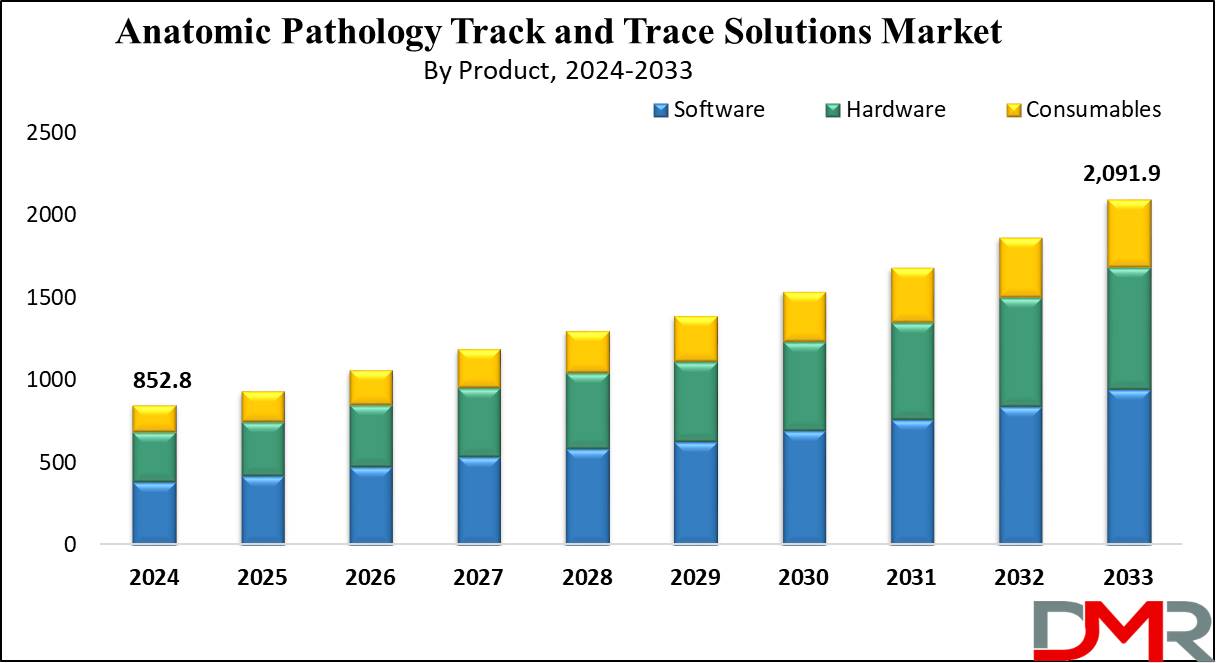
Anatomic Pathology Track And Trace Solutions Market

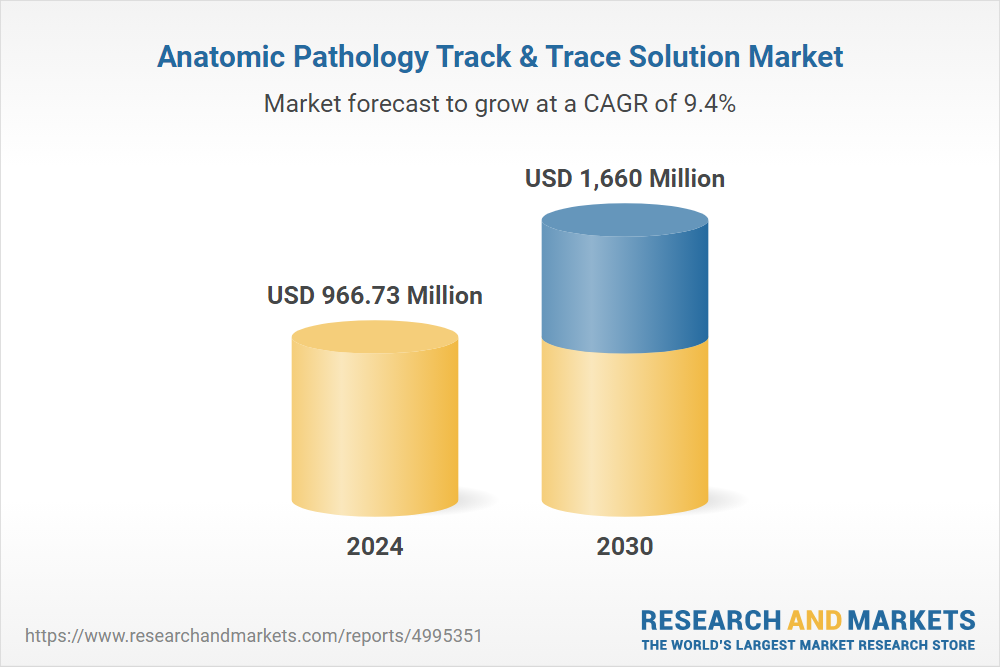
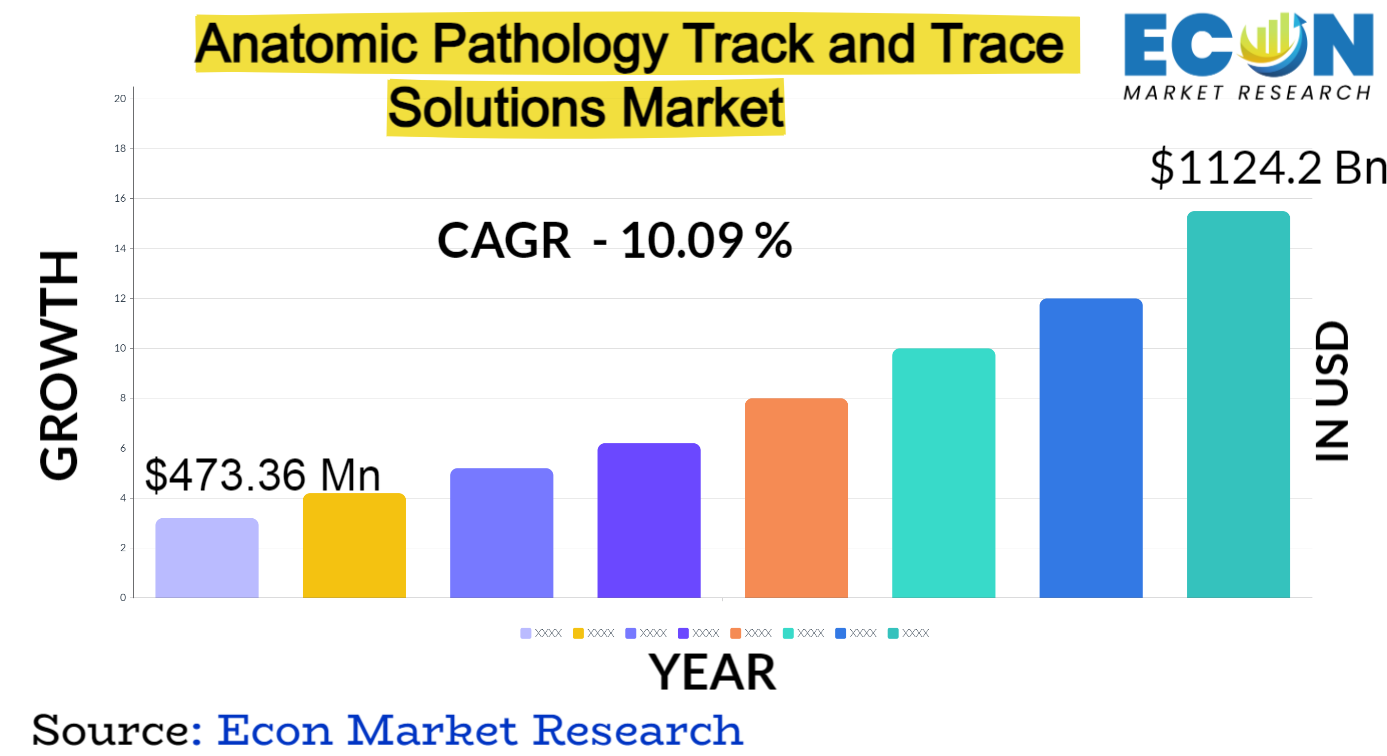
The anatomic pathology track and trace solutions market is experiencing significant growth, driven by increasing diagnostic testing volumes, stringent regulatory requirements, and the growing need for accurate and efficient sample management in healthcare facilities.
This burgeoning market plays a vital role in ensuring patient safety, reducing errors, and optimizing workflows within pathology laboratories. It impacts diagnostic accuracy and turnaround times, which ultimately affect patient care.
What are Anatomic Pathology Track and Trace Solutions?
Anatomic pathology track and trace solutions encompass a range of technologies and software systems used to meticulously monitor and manage tissue specimens throughout the entire pathology workflow.
These solutions are designed to prevent sample misidentification, ensure chain of custody, and provide comprehensive data on specimen location and processing history. They improve efficiency, reduce errors, and facilitate regulatory compliance.
Market Drivers and Trends
Several key factors are propelling the growth of this market. The increasing prevalence of cancer and other chronic diseases is driving the demand for diagnostic testing.
Furthermore, regulatory mandates from organizations like the College of American Pathologists (CAP) and other accrediting bodies require laboratories to implement robust tracking systems.
Another major driver is the growing adoption of laboratory automation, which necessitates integrated track and trace solutions to ensure seamless workflow management. Laboratories are increasingly focused on improving efficiency and reducing errors through automation.
Key Technologies and Components
The anatomic pathology track and trace solutions market encompasses various technologies, including barcode and RFID (Radio-Frequency Identification) systems.
Software platforms for sample tracking, data management, and reporting are also essential components. These platforms provide real-time visibility into the location and status of each specimen.
Additionally, specialized labels and consumables designed to withstand the harsh chemical environments in pathology laboratories play a critical role in maintaining data integrity.
Regional Market Analysis
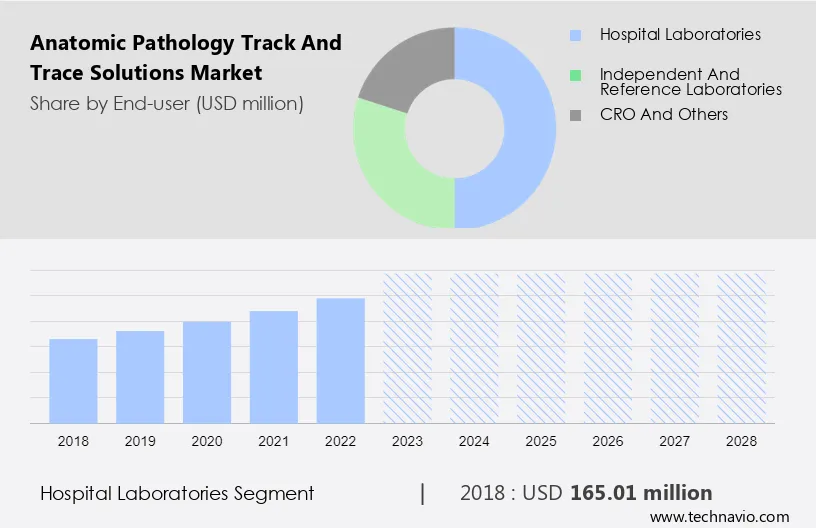
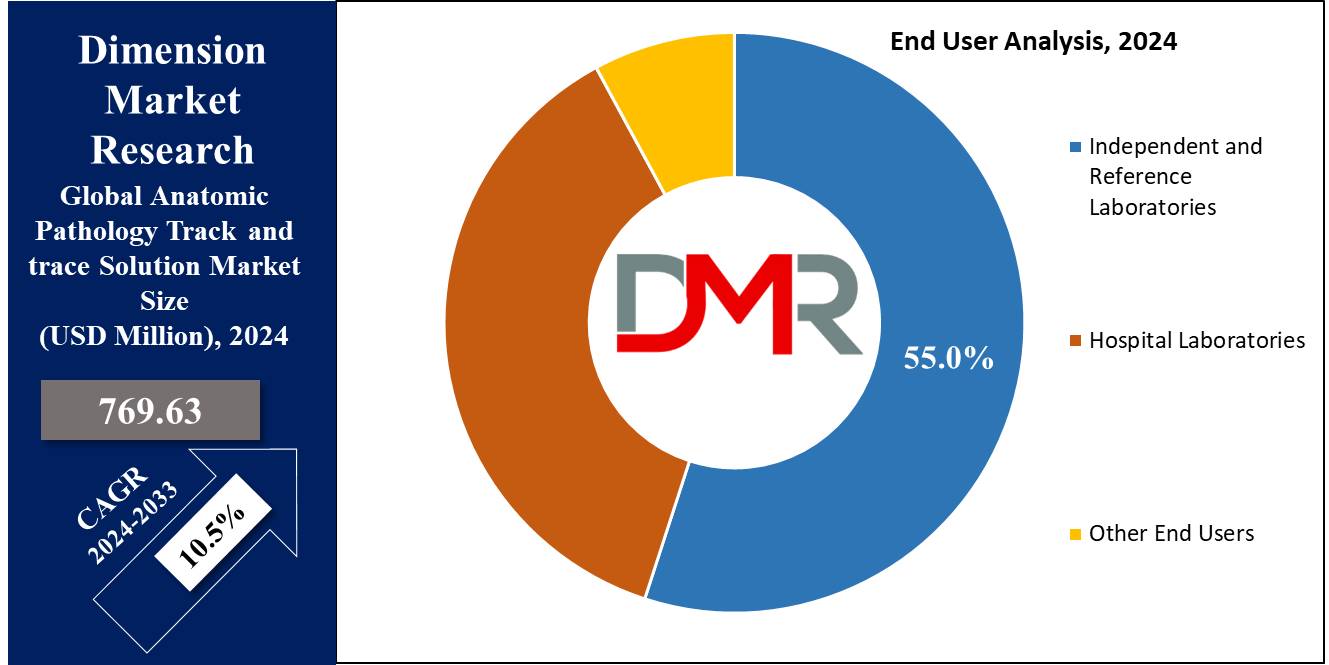
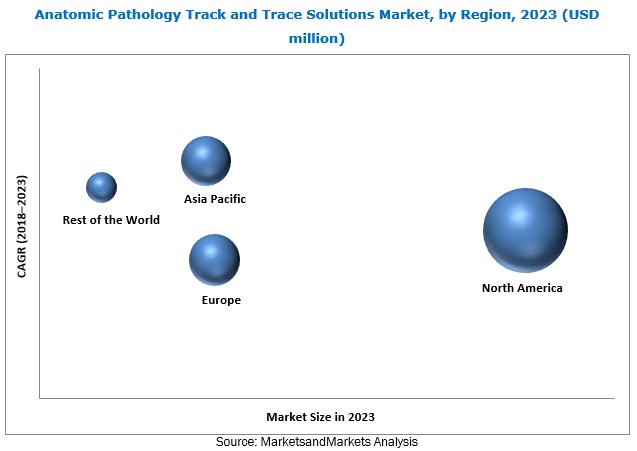
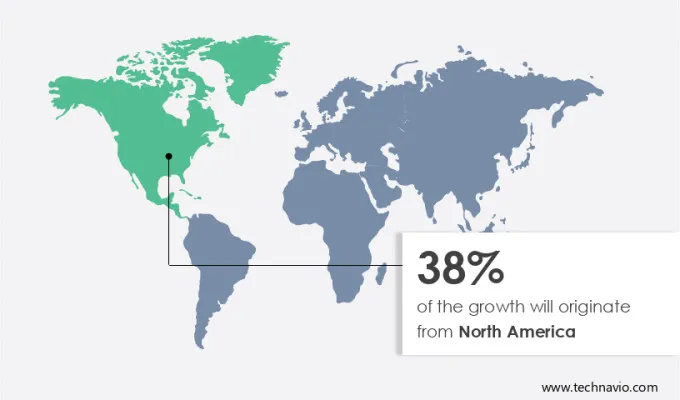
North America currently holds the largest share of the anatomic pathology track and trace solutions market. This dominance is attributed to the presence of advanced healthcare infrastructure and stringent regulatory environments.
Europe is also a significant market, driven by similar factors. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period.
This rapid growth is fueled by increasing healthcare expenditure, rising awareness of advanced diagnostic technologies, and the expansion of healthcare facilities in countries like China and India.
Key Players in the Market
The anatomic pathology track and trace solutions market is moderately competitive, with a mix of established players and emerging companies. Some of the leading companies include Thermo Fisher Scientific, Danaher Corporation (specifically through its subsidiaries like Leica Biosystems), and Sectra AB.
These companies offer a comprehensive range of products and services, including hardware, software, and consumables. Competition is primarily based on product innovation, technological advancements, and the ability to provide integrated solutions.
Other notable players in the market include Sunquest Information Systems, Cerner Corporation, and smaller, specialized companies focusing on niche applications.
Challenges and Opportunities
Despite the promising growth prospects, the anatomic pathology track and trace solutions market faces certain challenges. High implementation costs can be a barrier for smaller laboratories.
The need for seamless integration with existing laboratory information systems (LIS) can also pose a challenge. Data security and privacy concerns are paramount and require robust security measures.
However, significant opportunities exist for market expansion. The increasing adoption of digital pathology and artificial intelligence (AI) presents new avenues for integrating track and trace solutions. The development of cloud-based solutions offers scalability and cost-effectiveness.
Impact on Patient Care
The adoption of anatomic pathology track and trace solutions has a direct and positive impact on patient care. By reducing errors in sample identification and processing, these solutions enhance diagnostic accuracy.
Improved workflow efficiency translates into faster turnaround times for test results, enabling clinicians to make timely and informed treatment decisions. Enhanced traceability and data integrity contribute to improved patient safety and reduced risk of misdiagnosis.
Ultimately, these solutions play a critical role in ensuring that patients receive the right diagnosis and treatment, leading to better health outcomes.
The Future of Track and Trace in Anatomic Pathology
The future of anatomic pathology track and trace solutions is likely to be characterized by increased integration with digital pathology and AI technologies. Cloud-based solutions will become more prevalent, offering enhanced scalability and accessibility.
The development of more sophisticated algorithms for predictive analytics will enable laboratories to proactively identify and address potential errors. Furthermore, increased emphasis on data security and privacy will drive the adoption of advanced security measures.
As the demand for diagnostic testing continues to rise, anatomic pathology track and trace solutions will play an increasingly critical role in ensuring the accuracy, efficiency, and safety of pathology laboratories worldwide.




.png)