Bellabeat.com Signs Of Chemical Pregnancy At 4 Weeks

The digital health company Bellabeat is facing scrutiny following the surfacing of concerns surrounding the accuracy and potential misinterpretation of their tracking data, specifically in relation to early pregnancy detection. Several users have reported receiving indications of a potential chemical pregnancy around the four-week mark, only to later discover that they were not pregnant.
This situation raises crucial questions about the reliability of using wearable technology for sensitive health monitoring, the responsibility of companies providing such data, and the emotional impact on individuals relying on these tools for family planning.
The Bellabeat Promise and its Challenges
Bellabeat, known for its wellness trackers and associated app, aims to empower women with data-driven insights into their health, including cycle tracking and fertility awareness. The company’s devices monitor various physiological metrics, such as basal body temperature (BBT), heart rate variability (HRV), and sleep patterns, to identify potential fertile windows and even suggest possible pregnancy.
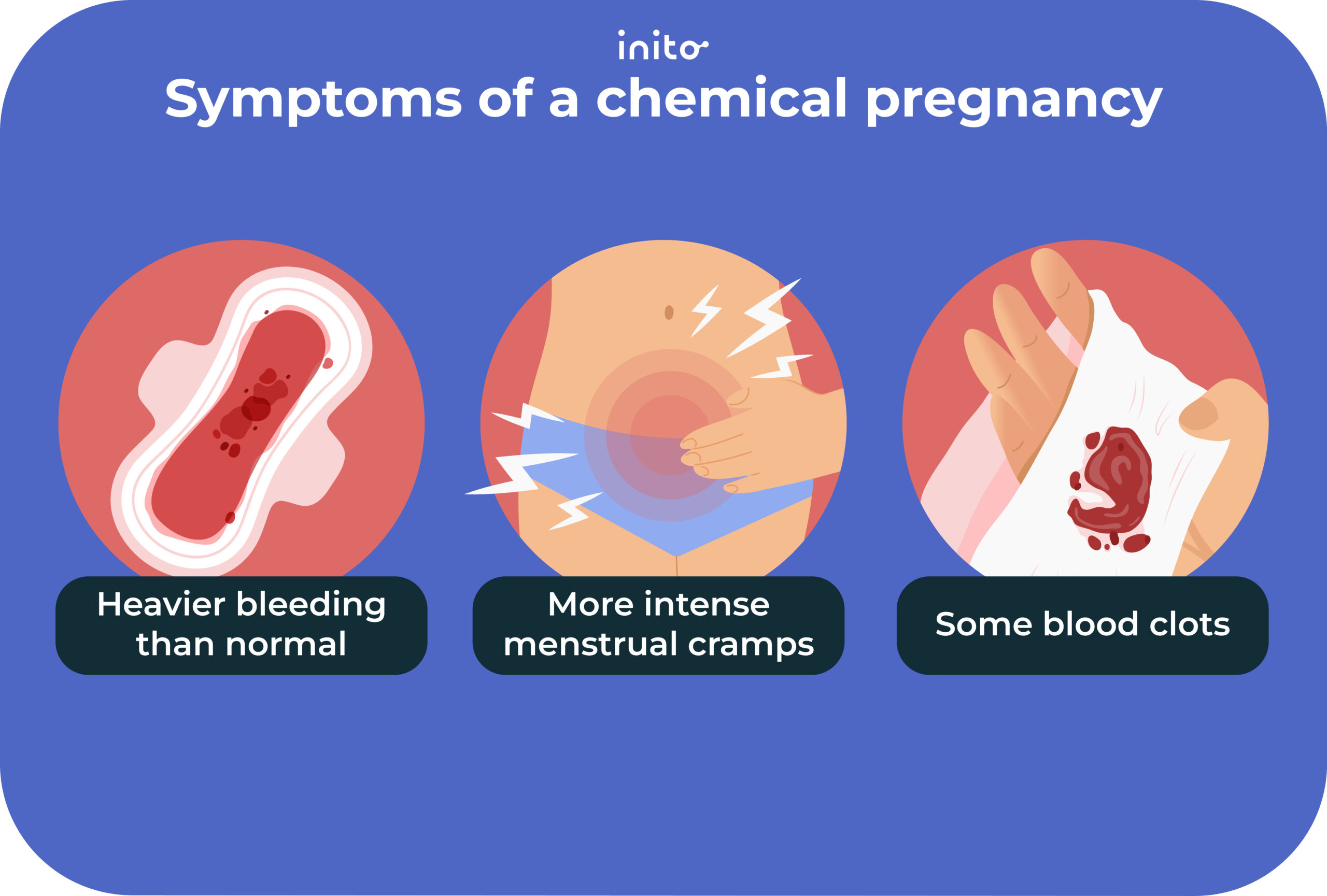
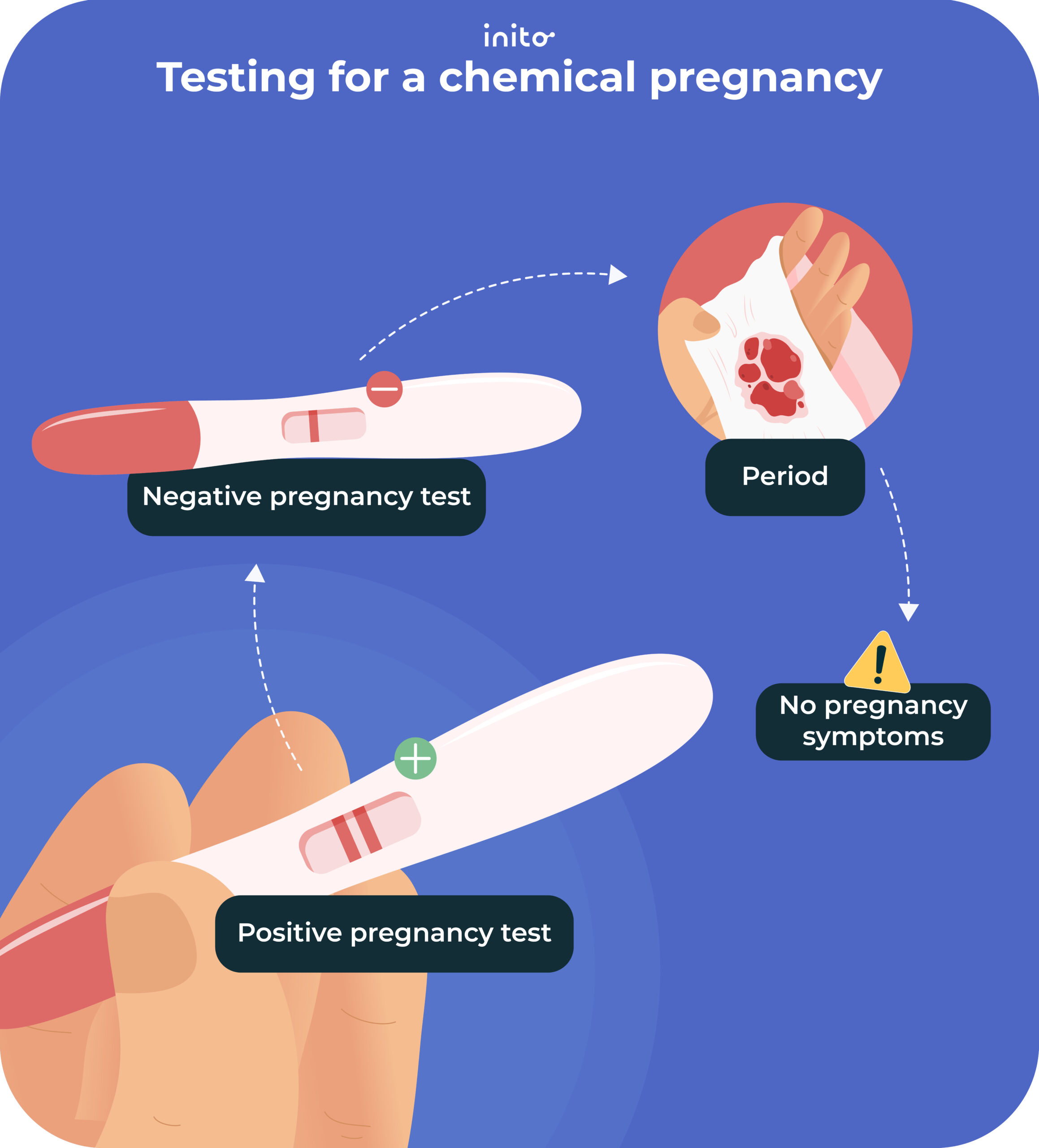
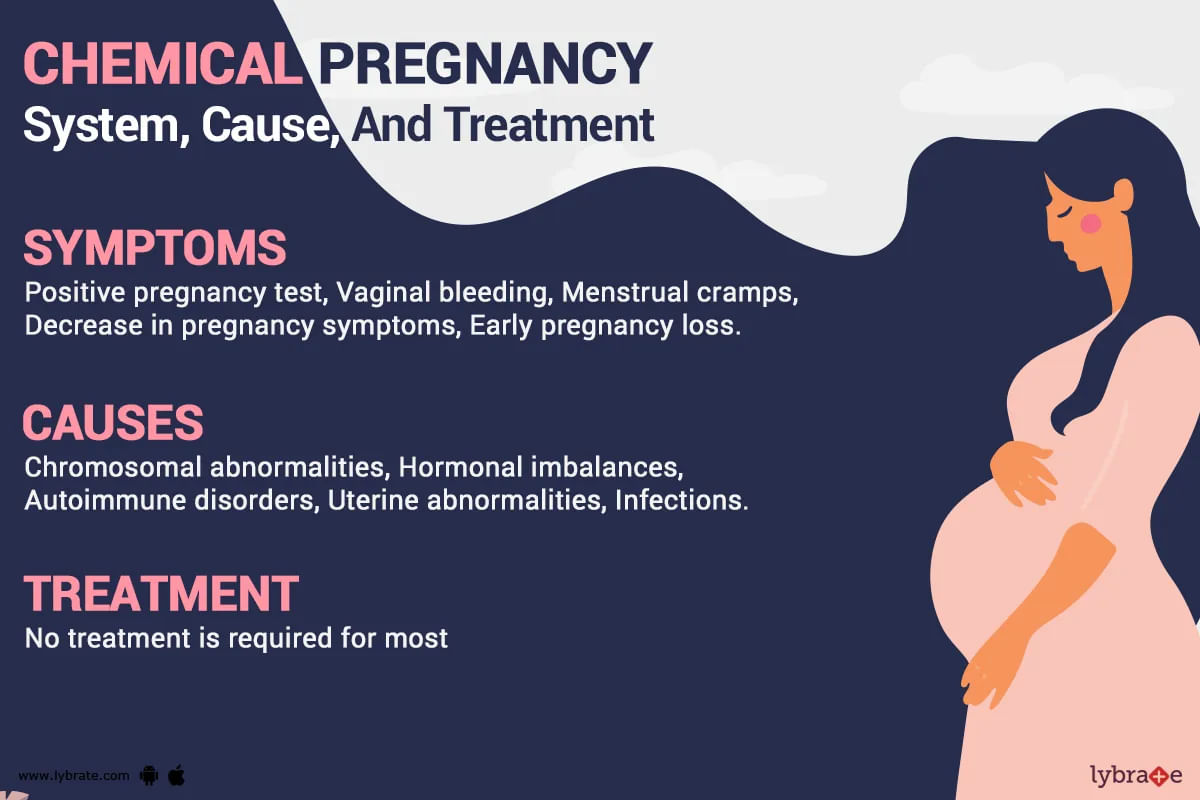
The challenge arises when algorithms designed to detect early pregnancy indicators, such as a sustained increase in BBT, are misinterpreted or lead to false positives. A "chemical pregnancy" refers to a very early pregnancy loss that occurs shortly after implantation, often before a clinical pregnancy can be detected by ultrasound. A positive pregnancy test may occur due to the presence of human chorionic gonadotropin (hCG), but the pregnancy does not progress.
Reports of Misleading Data
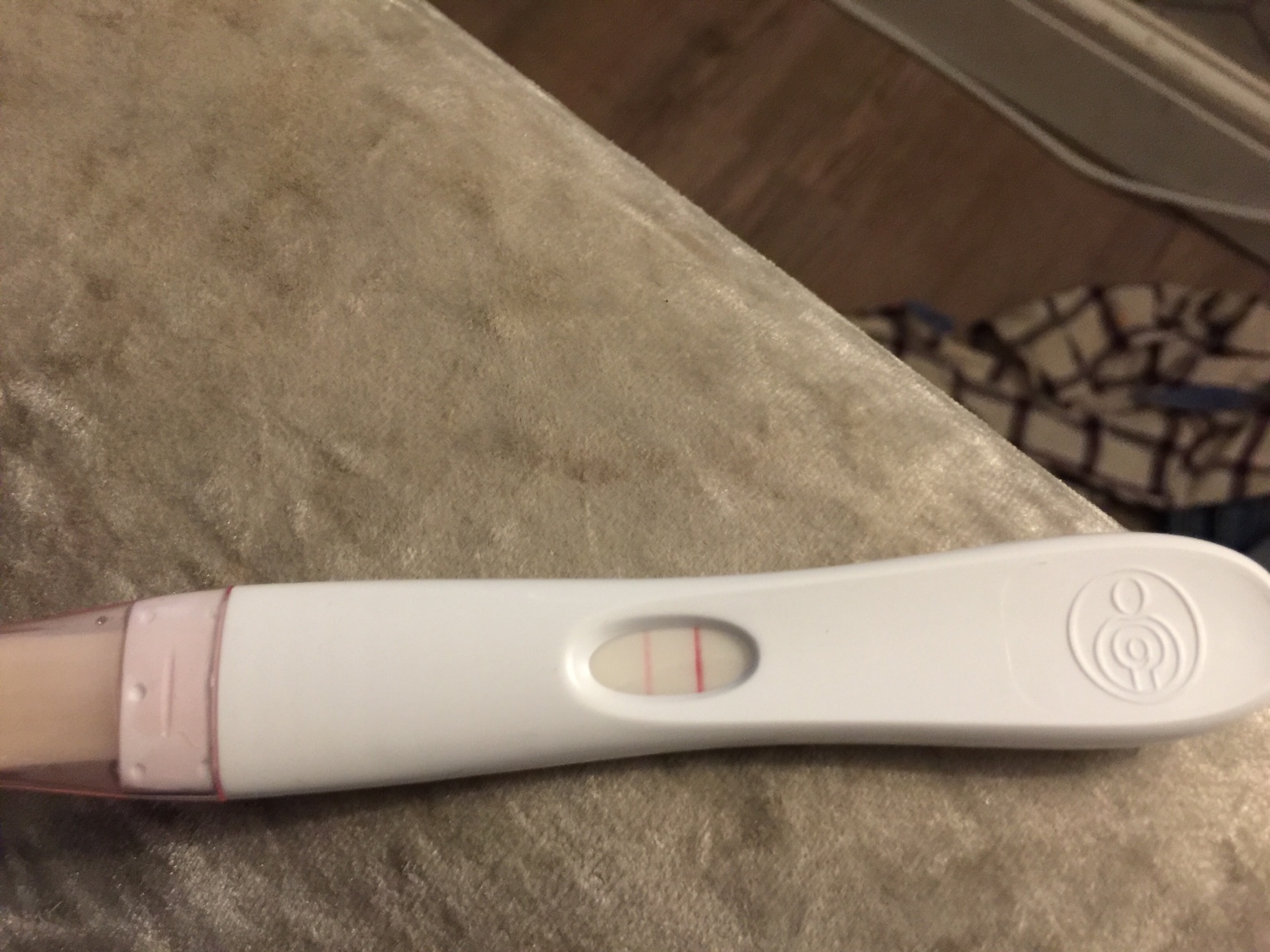
Numerous anecdotal reports have emerged across online forums and social media platforms detailing instances where Bellabeat data allegedly indicated a potential chemical pregnancy around week four. These reports often involve a period of heightened excitement and anticipation, followed by disappointment and emotional distress when subsequent tests or medical evaluations confirm the absence of a viable pregnancy.
One user, posting in an online fertility forum, described the experience as "a rollercoaster of emotions," stating that the Bellabeat app suggested a pregnancy based on BBT data, leading her to believe she was pregnant for several days before a negative blood test confirmed otherwise.
Expert Perspectives on Wearable Technology and Early Pregnancy Detection
Medical professionals are cautious about relying solely on wearable technology for diagnosing or confirming pregnancy, especially in the very early stages. Dr. Emily Carter, a reproductive endocrinologist, emphasizes that "while wearable devices can provide valuable data, they are not substitutes for medical evaluation and testing."
"BBT can be influenced by various factors, including sleep disturbances, stress, and illness," Dr. Carter explains, adding that "relying solely on this metric for pregnancy detection can lead to inaccuracies and unnecessary emotional distress."
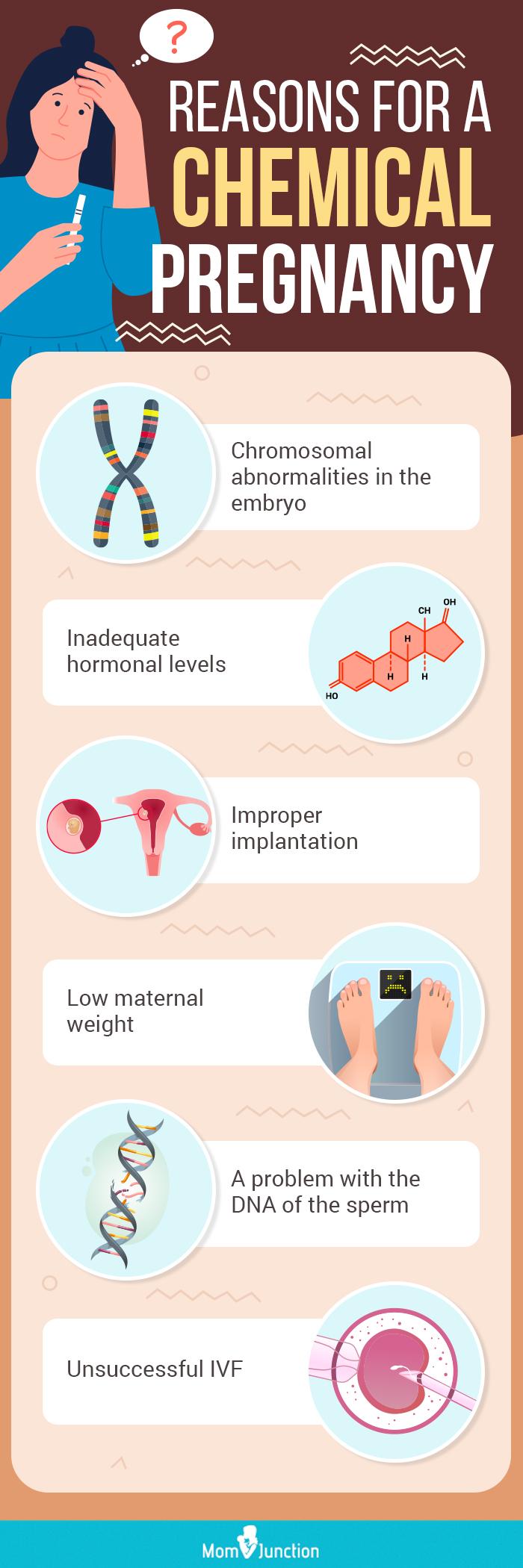
Furthermore, Dr. David Lee, an OB/GYN, highlights the limitations of current algorithms used in wearable devices for detecting chemical pregnancies. "These algorithms are often based on generalized data and may not accurately reflect individual physiological variations," he notes. This can lead to false positives, particularly in cases where other factors are influencing the tracked metrics.
Bellabeat's Response and Recommendations
Bellabeat has acknowledged the concerns raised by users and emphasized that their devices are intended for general wellness tracking and fertility awareness, not for diagnosing medical conditions. The company's official statement, released earlier this week, stated: "Our devices and app provide insights into a woman's health and cycle, but they are not intended to replace medical advice or testing."
"We encourage users to consult with their healthcare providers for any concerns regarding their health or potential pregnancy," the statement continued.
Bellabeat also outlined steps they are taking to improve the clarity of information provided to users. These include revising the app's interface to clearly state the limitations of early pregnancy detection and providing more comprehensive educational resources on interpreting the data collected by their devices.
The Ethical Considerations and the Future of Fertility Tech
The Bellabeat situation underscores the broader ethical considerations surrounding the use of technology in reproductive health. Companies offering fertility tracking and pregnancy prediction tools have a responsibility to ensure that their products are accurate, transparent, and do not cause undue emotional distress.
Experts suggest that regulatory oversight may be needed to establish standards for the accuracy and reliability of these devices, as well as to ensure that users are adequately informed about their limitations. Moreover, mental health support should be available to users experiencing emotional distress related to false positives or other inaccuracies in fertility tracking data.
As technology continues to evolve, wearable devices have the potential to play a significant role in reproductive health. However, it is crucial that these tools are developed and used responsibly, with a focus on accuracy, transparency, and the well-being of the individuals relying on them.