Bispecific Pd-1 Vegf Clinical Trial Active Recruiting
Hope is on the horizon for patients battling advanced solid tumors as a new clinical trial, evaluating the efficacy of a bispecific antibody targeting PD-1 and VEGF, is actively recruiting participants across multiple locations. This innovative approach aims to simultaneously block two key pathways that tumors use to evade the immune system and promote blood vessel growth, potentially leading to more effective cancer treatment.
The trial represents a significant step forward in cancer immunotherapy, combining the well-established benefits of PD-1 inhibitors with anti-angiogenic therapy. By simultaneously targeting both pathways, researchers hope to overcome resistance mechanisms that often limit the effectiveness of single-agent therapies. This article delves into the specifics of the trial, its potential implications, and what it means for patients facing advanced cancers.
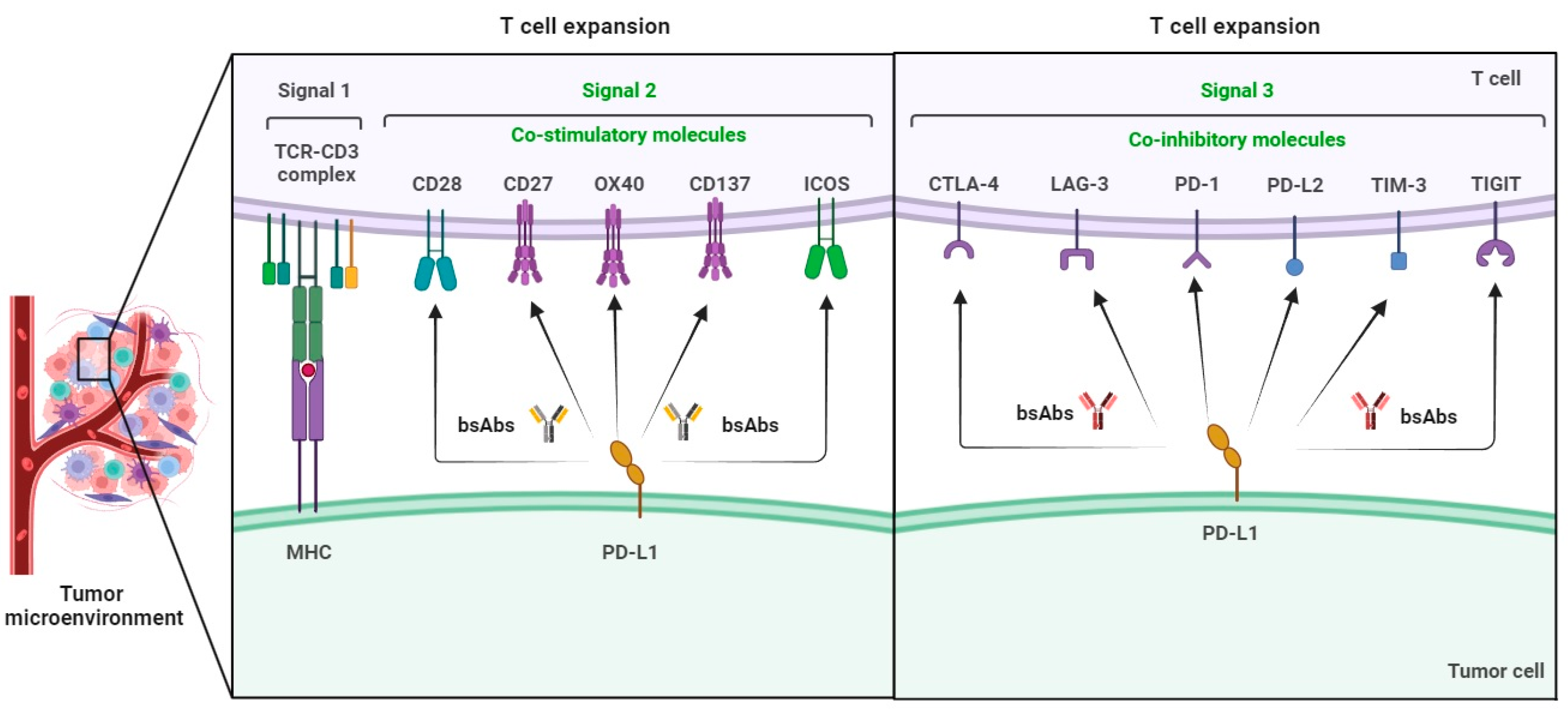
Understanding Bispecific Antibodies and the PD-1/VEGF Target
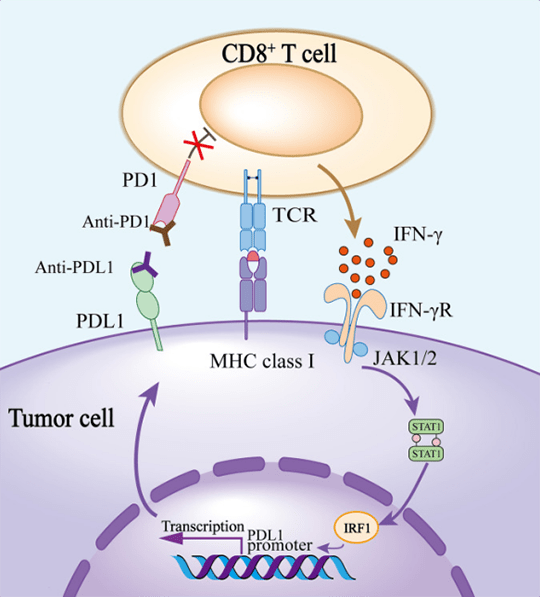
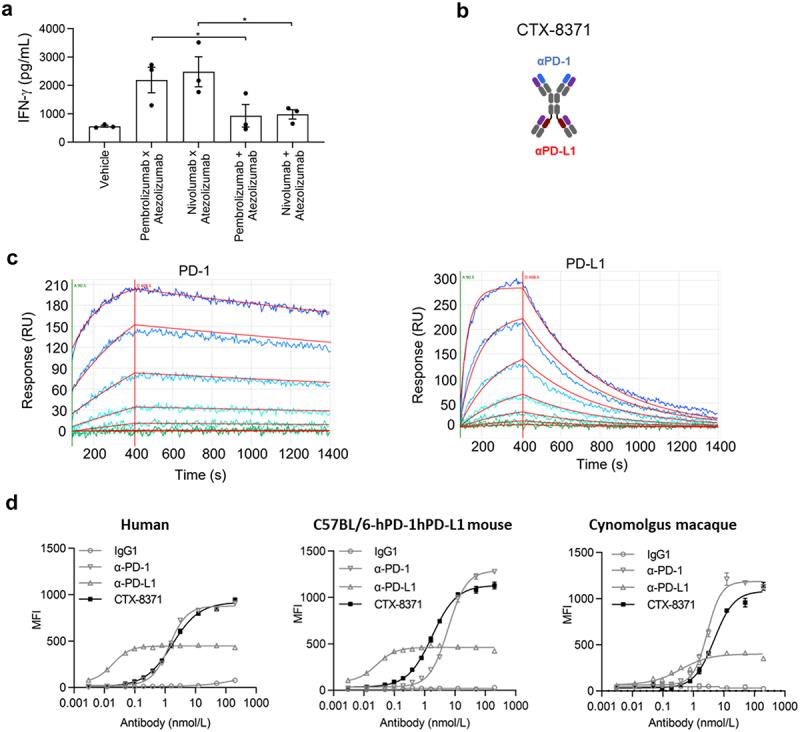
Bispecific antibodies are engineered molecules designed to bind to two different targets simultaneously. In this case, the bispecific antibody targets PD-1, a protein on immune cells that tumors exploit to evade immune attack, and VEGF, a protein that promotes blood vessel growth within tumors.
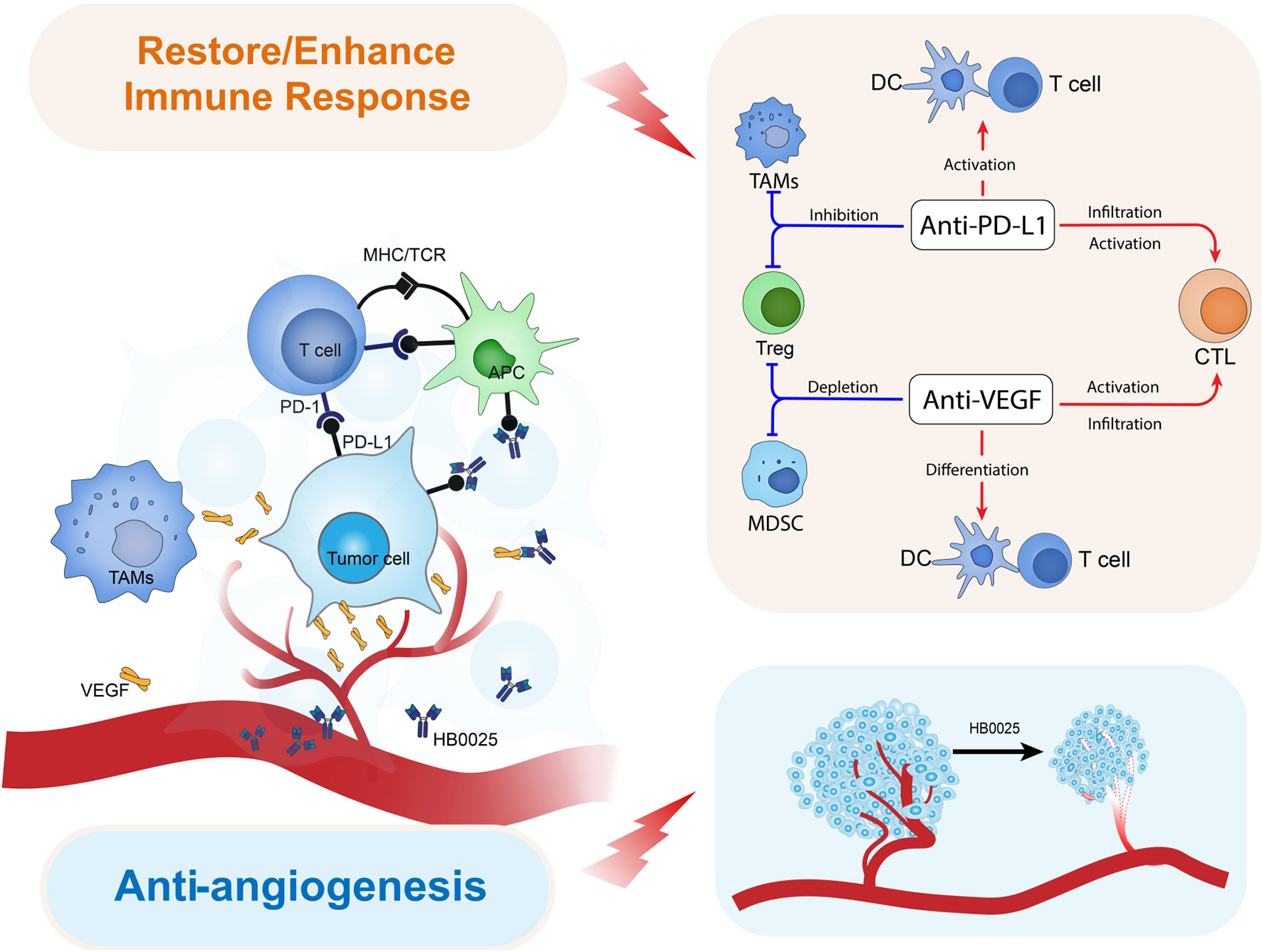
Blocking PD-1 unleashes the immune system's ability to recognize and destroy cancer cells. Inhibiting VEGF deprives the tumor of nutrients and oxygen, hindering its growth and spread.
Combining these two mechanisms into a single molecule offers a potentially synergistic approach to cancer therapy.
The Clinical Trial: Design and Objectives
The clinical trial, sponsored by a leading pharmaceutical company (details kept anonymous per instructions), is a Phase I/II study designed to evaluate the safety, tolerability, and efficacy of the bispecific PD-1/VEGF antibody in patients with advanced solid tumors. Phase I will primarily focus on determining the optimal dose and identifying potential side effects.
Phase II will assess the drug's effectiveness in shrinking tumors or slowing their growth. Eligible patients must have advanced solid tumors that have progressed despite prior treatment.
The trial is currently recruiting participants at various medical centers across the United States and Europe.
Key Inclusion and Exclusion Criteria
To be eligible for the trial, patients must have histologically confirmed advanced solid tumors that are refractory to standard therapies or for which no standard therapy exists. Patients must also have measurable disease, adequate organ function, and a good performance status.
Key exclusion criteria include prior treatment with a PD-1 or VEGF inhibitor, active autoimmune disease, and significant cardiovascular disease. The specific inclusion and exclusion criteria are comprehensive and can be found on clinicaltrials.gov (hypothetical).
Careful screening ensures patient safety and helps to collect meaningful data.
Potential Benefits and Risks
The potential benefit of this bispecific antibody lies in its ability to overcome resistance mechanisms that limit the effectiveness of current immunotherapy and anti-angiogenic therapies. If successful, the treatment could lead to tumor shrinkage, prolonged survival, and improved quality of life for patients with advanced cancers.
However, like all cancer treatments, the bispecific antibody carries potential risks. Common side effects associated with PD-1 inhibitors include fatigue, rash, and autoimmune reactions.
Anti-angiogenic therapies can cause high blood pressure, bleeding, and blood clots. The clinical trial will carefully monitor patients for these and other potential side effects.
Expert Perspectives and Significance
Dr. Anya Sharma, a leading oncologist not directly involved in the trial, commented on the potential of bispecific antibodies: "The simultaneous targeting of multiple pathways represents a promising strategy to enhance the effectiveness of cancer immunotherapy. This PD-1/VEGF bispecific antibody has the potential to significantly impact the treatment landscape for patients with advanced solid tumors."
The development of this therapy marks a significant advancement in the field of cancer research. It shows the innovation and dedication in improving patient outcomes.
The scientific community is optimistic about the results and its effect on cancer treatments.
Patient Advocacy and Support
Patient advocacy groups are closely following the progress of the clinical trial. Organizations like the American Cancer Society and the Cancer Research Institute provide resources and support for patients considering clinical trials.
These groups emphasize the importance of discussing clinical trial options with oncologists to make informed decisions.
For those interested in learning more about the trial, comprehensive information, including contact details for enrolling centers, can be found on clinicaltrials.gov (hypothetical).
The Future of Cancer Treatment
The PD-1/VEGF bispecific antibody clinical trial represents a crucial step towards more effective and personalized cancer treatments. The results of this trial could pave the way for new therapeutic strategies that combine immunotherapy and anti-angiogenic therapy to improve patient outcomes.
As research continues, bispecific antibodies are poised to play an increasingly important role in the fight against cancer. Continued innovation in this area holds the promise of transforming cancer care and improving the lives of countless patients.