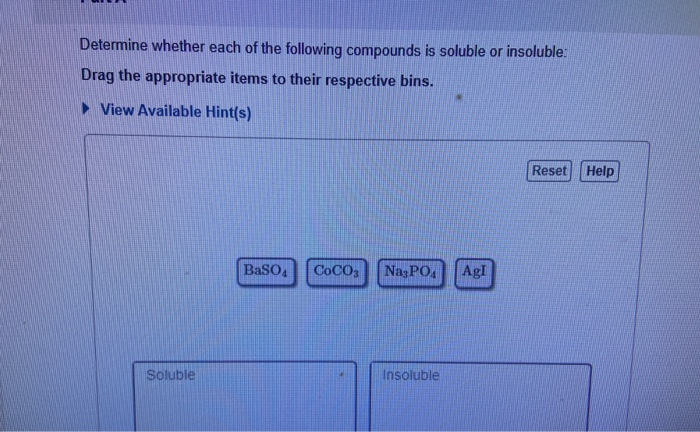
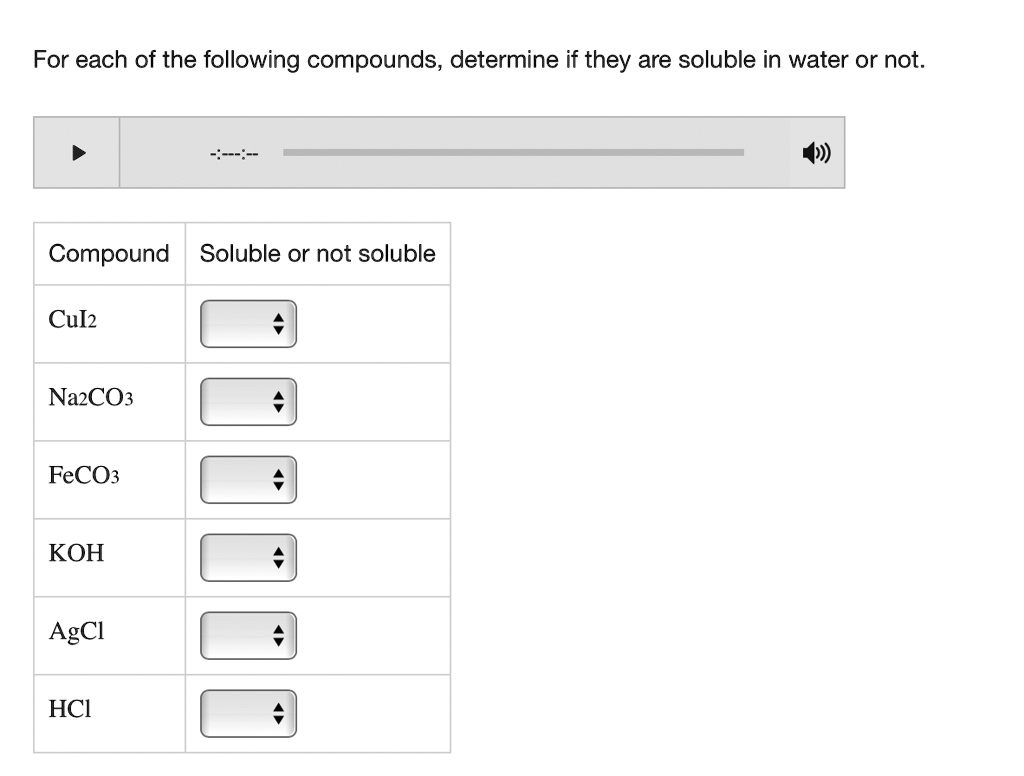
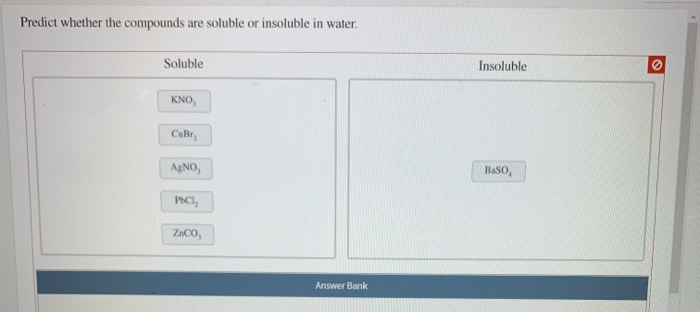
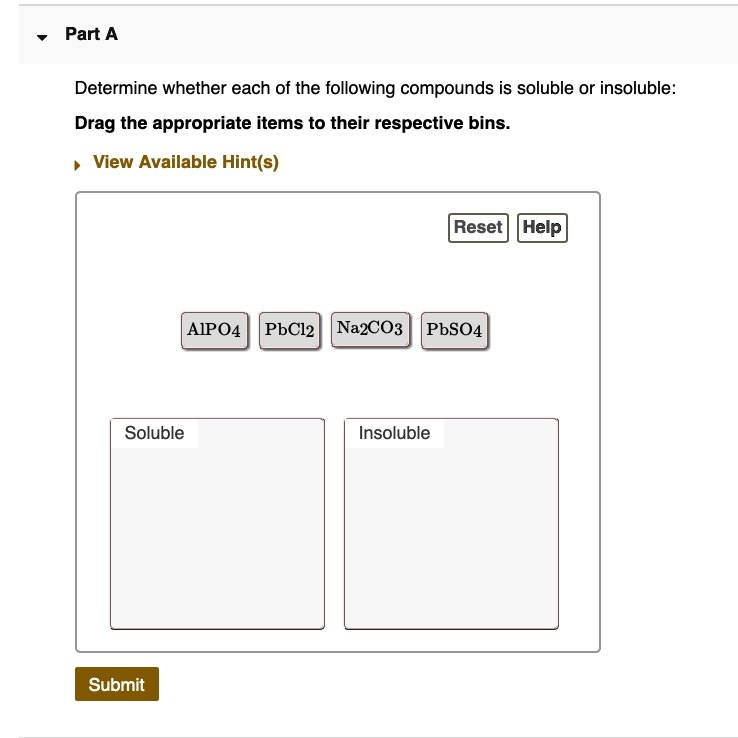
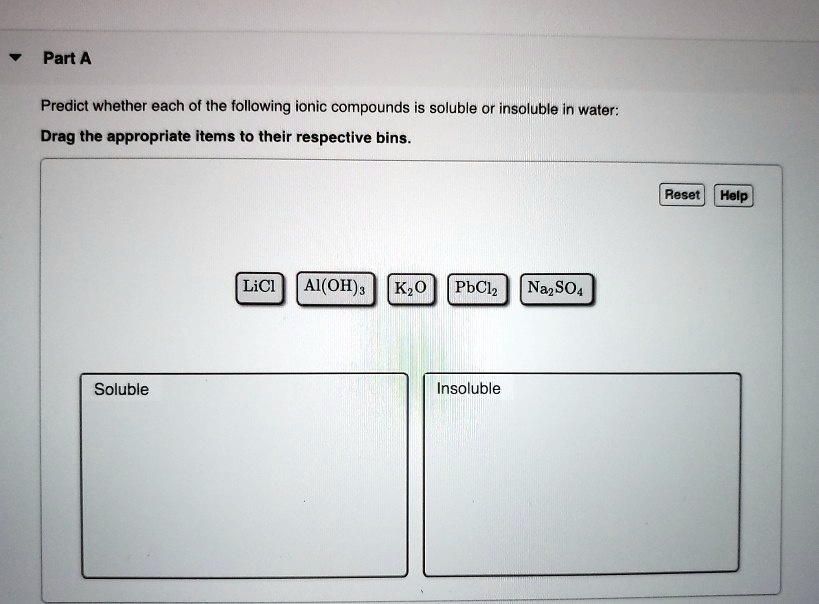
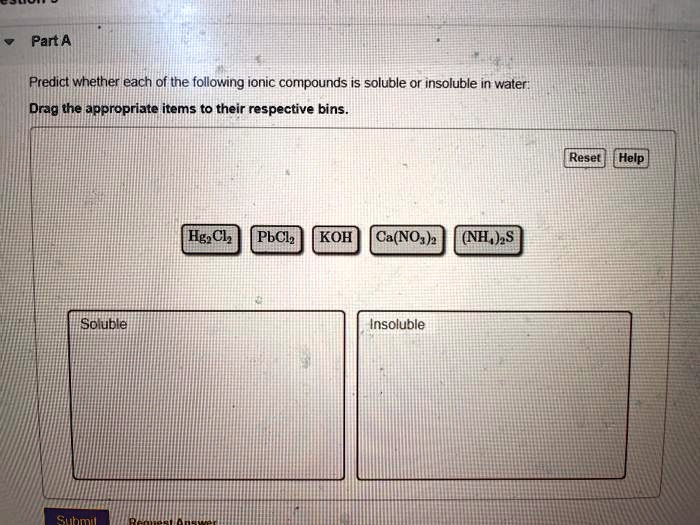
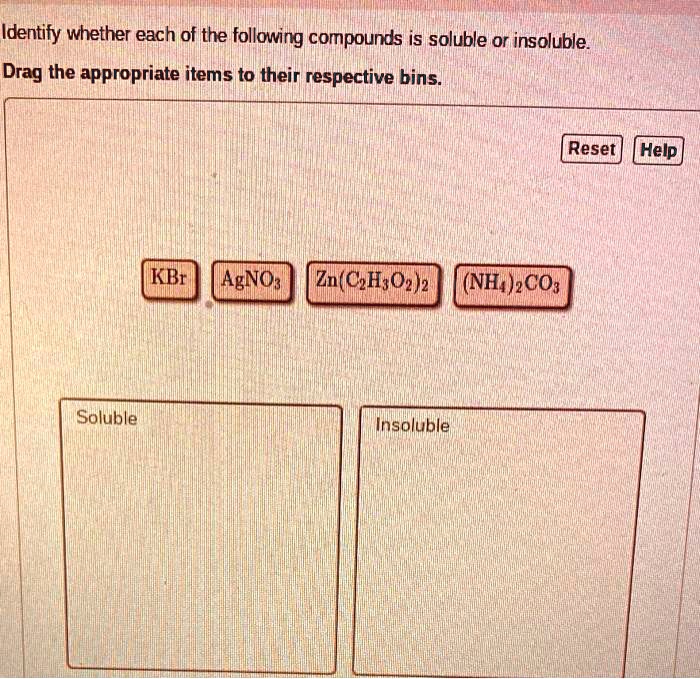
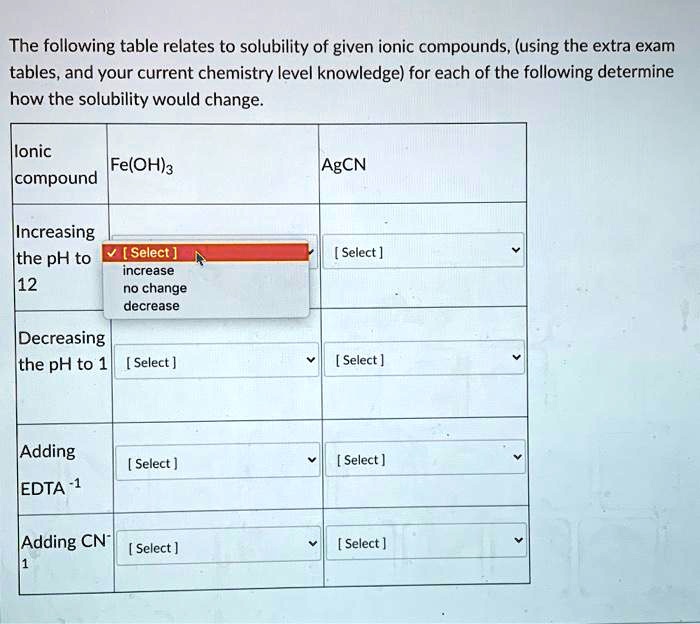
Determine Whether Each Of The Following Compounds Is Soluble

Imagine a bustling chemistry lab, beakers bubbling gently on hot plates, the air thick with the subtle scent of various reagents. Sunlight streams through the windows, illuminating dust motes dancing in the air as a student meticulously adds a pinch of crystalline powder to a flask of water. The question hanging in the air: will it dissolve, or will it remain stubbornly undissolved at the bottom?
Solubility, the ability of a substance to dissolve in a solvent, is a fundamental concept in chemistry with widespread implications, from the development of new drugs to the treatment of wastewater. This article explores the factors governing solubility, equipping readers with the knowledge to predict whether a given compound will dissolve in a particular solvent.
The Golden Rule: "Like Dissolves Like"
At its core, solubility is governed by the principle that "like dissolves like." This means that polar solvents, such as water, tend to dissolve polar solutes, while nonpolar solvents, like hexane, are better at dissolving nonpolar solutes.
This behavior stems from the intermolecular forces that hold molecules together.
Let's dive into it.
Polarity: The Key to Understanding Solubility
Polarity arises when there's an uneven distribution of electrons within a molecule, creating partial positive and negative charges.
Water (H2O), with its bent shape and highly electronegative oxygen atom, is a prime example of a polar solvent.
Nonpolar molecules, on the other hand, have an even distribution of electrons.
Consider methane (CH4), a symmetrical molecule where the electron density is evenly distributed across the carbon-hydrogen bonds. It is, therefore, nonpolar.
Ionic Compounds and Their Solubility
Ionic compounds, formed by the electrostatic attraction between positively charged cations and negatively charged anions, are generally soluble in polar solvents like water.
Water molecules surround the ions, effectively neutralizing their charges and dispersing them throughout the solution.
This process is called solvation or, in the case of water, hydration.
However, not all ionic compounds are created equal in terms of solubility.
Factors like the lattice energy of the crystal and the hydration energy of the ions play crucial roles.
High lattice energy (stronger attraction between ions) makes it harder to break apart the crystal, while high hydration energy (stronger attraction between ions and water) promotes dissolution.
General solubility rules for ionic compounds provide a helpful guide:
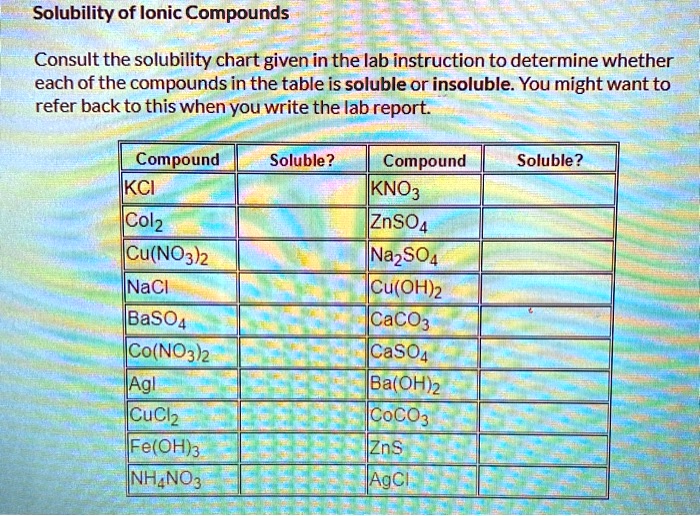
- Salts containing alkali metal ions (Li+, Na+, K+, Rb+, Cs+) and the ammonium ion (NH4+) are generally soluble.
- Salts containing nitrate (NO3-), acetate (CH3COO-), perchlorate (ClO4-) are generally soluble.
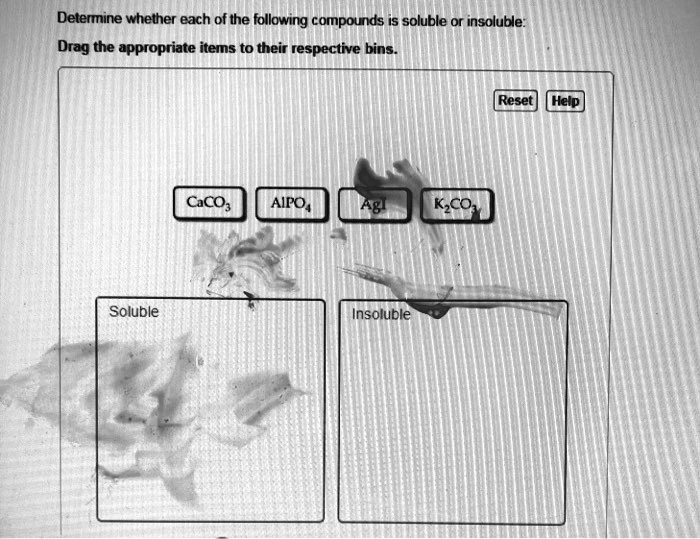
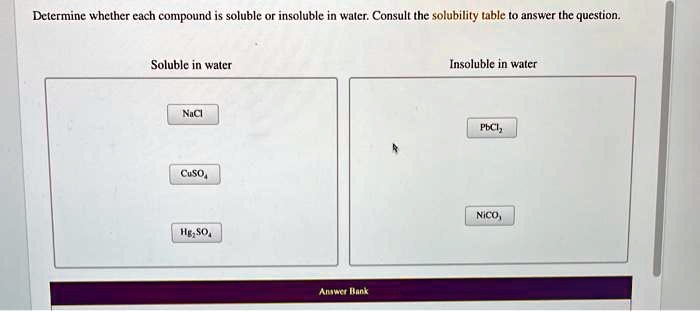
- Most chloride (Cl-), bromide (Br-), and iodide (I-) salts are soluble, except those of silver (Ag+), lead (Pb2+), and mercury(I) (Hg22+).
- Most sulfate (SO42-) salts are soluble, except those of barium (Ba2+), strontium (Sr2+), lead (Pb2+), and calcium (Ca2+).
- Most hydroxide (OH-) and sulfide (S2-) salts are insoluble, except those of alkali metals and ammonium. Calcium, strontium, and barium hydroxides are moderately soluble.
- Most carbonate (CO32-) and phosphate (PO43-) salts are insoluble, except those of alkali metals and ammonium.
Applying the Rules: Examples in Action
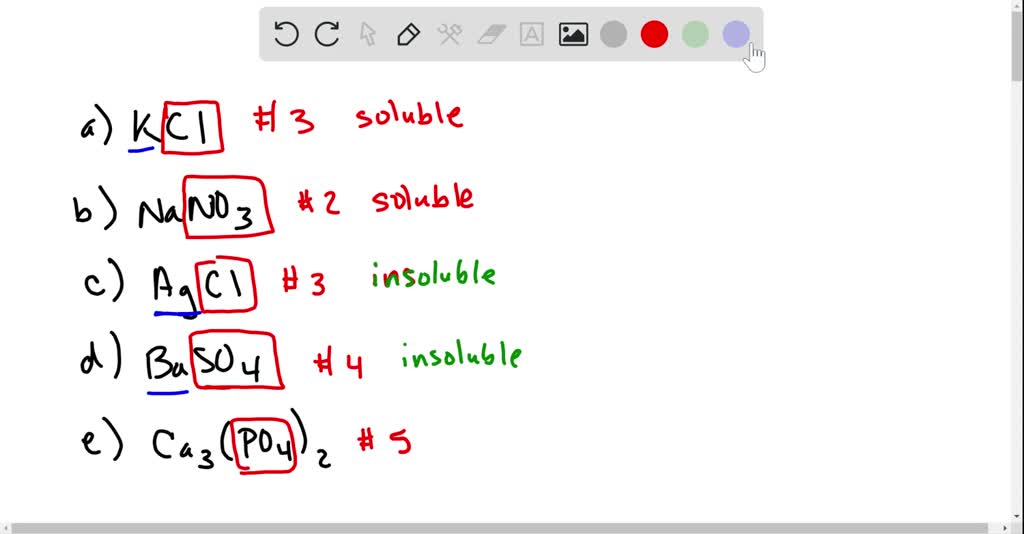
Let's apply these rules to some specific examples. Consider sodium chloride (NaCl), common table salt.
According to the rules, salts containing alkali metal ions (Na+) are generally soluble. Thus, NaCl is soluble in water.
Now, consider silver chloride (AgCl).
The rules state that most chloride salts are soluble except those of silver.
Therefore, AgCl is insoluble in water, forming a precipitate.
What about copper(II) sulfate (CuSO4)?
Most sulfate salts are soluble. Therefore, CuSO4 is soluble in water.
These examples demonstrate how the general solubility rules can be used to predict the solubility of ionic compounds.
However, it's essential to remember that these rules are just guidelines; there are always exceptions.
Beyond Ionic Compounds: Solubility of Covalent Compounds
The solubility of covalent compounds is more complex and depends heavily on the polarity of the molecule and the solvent.
Polar covalent compounds, like ethanol (CH3CH2OH), tend to be soluble in polar solvents due to the presence of polar bonds and the ability to form hydrogen bonds with water.
Nonpolar covalent compounds, such as hexane (C6H14), are soluble in nonpolar solvents due to weak Van der Waals forces.
The presence of hydroxyl (-OH) groups in a molecule generally increases its solubility in water due to hydrogen bonding.
Conversely, long hydrocarbon chains decrease solubility in water as they are nonpolar.
The balance between polar and nonpolar regions within a molecule determines its overall solubility.
Factors Affecting Solubility: Temperature and Pressure
Temperature and pressure can also significantly influence solubility.
For most solid solutes, solubility increases with increasing temperature.
This is because higher temperatures provide more energy to break the bonds holding the solute together.
The effect of pressure on solubility is most pronounced for gases.
According to Henry's Law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid.
Increasing the pressure forces more gas molecules into the liquid, increasing its solubility.
Solubility in Action: Real-World Applications
Solubility plays a critical role in a wide range of applications.
In medicine, the solubility of a drug determines how well it is absorbed into the bloodstream and distributed throughout the body.
Pharmaceutical companies carefully formulate drugs to optimize their solubility and bioavailability.
In environmental science, solubility is crucial for understanding the fate and transport of pollutants in water and soil.
Soluble pollutants can easily contaminate water sources, posing risks to human health and the environment.
Understanding solubility helps in developing effective remediation strategies.
In industry, solubility is used in various processes, from the extraction of valuable metals from ores to the production of detergents and paints.
The ability to control solubility is essential for many chemical processes.
Whether we realize it or not, solubility impacts our lives every day.
Conclusion: A Never-Ending Exploration
Predicting solubility isn't just about memorizing rules; it's about understanding the fundamental principles that govern molecular interactions.
While general solubility rules and the "like dissolves like" principle provide a solid foundation, the complexities of molecular structure and intermolecular forces often require a nuanced understanding.
Ultimately, solubility is a fascinating and dynamic area of study with far-reaching implications for science, technology, and society.