Diamond Is A Element Or Compound

Breaking: Confusion surrounding the fundamental nature of diamond has reached a fever pitch, with widespread debate erupting over whether it qualifies as an element or a compound. The scientific community is scrambling to clarify this foundational concept in chemistry.
This article addresses the urgent need to definitively state the composition of diamond, settling the ongoing dispute and providing a clear understanding for students, educators, and the general public. Misinformation online has fueled the confusion, necessitating immediate and accurate clarification.
Diamond's Composition: The Undisputed Truth
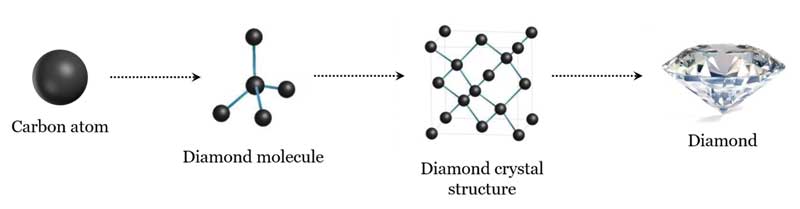
Diamond is unequivocally an element. It consists solely of carbon atoms arranged in a specific crystal lattice structure.
Unlike compounds, which are formed from two or more different elements chemically bonded together, diamonds contain only carbon.
This single-element composition is the key differentiating factor.
Understanding Elements vs. Compounds
An element is a pure substance that cannot be broken down into simpler substances by chemical means.
Carbon, oxygen, gold, and iron are all examples of elements.
A compound, conversely, is formed when two or more elements are chemically bonded in a fixed ratio.
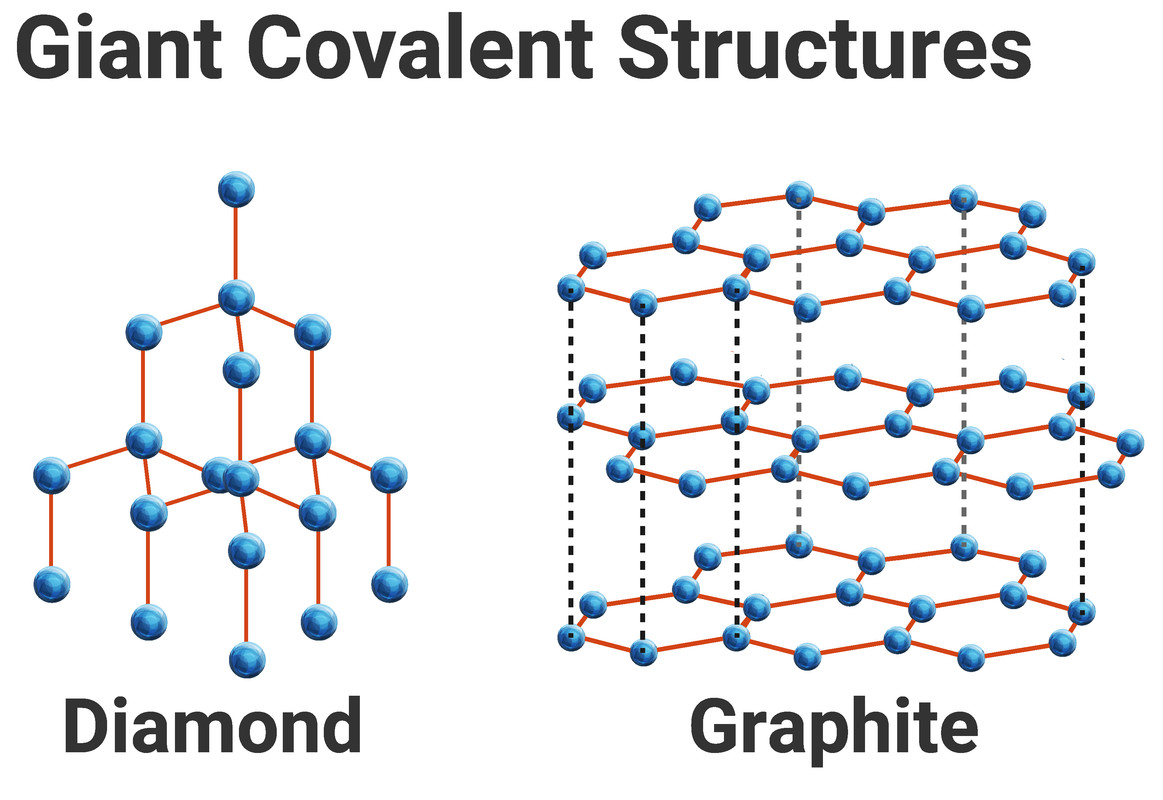
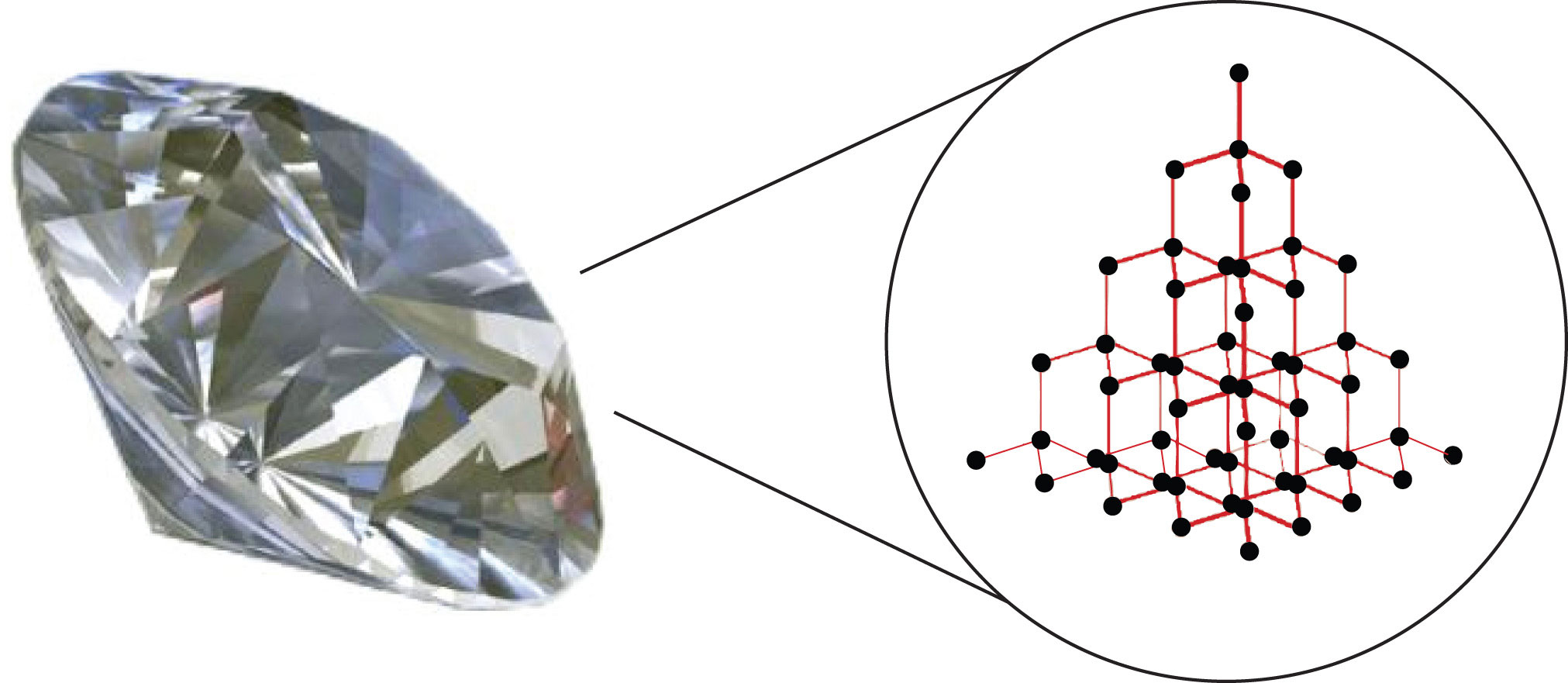
The Carbon Lattice Structure
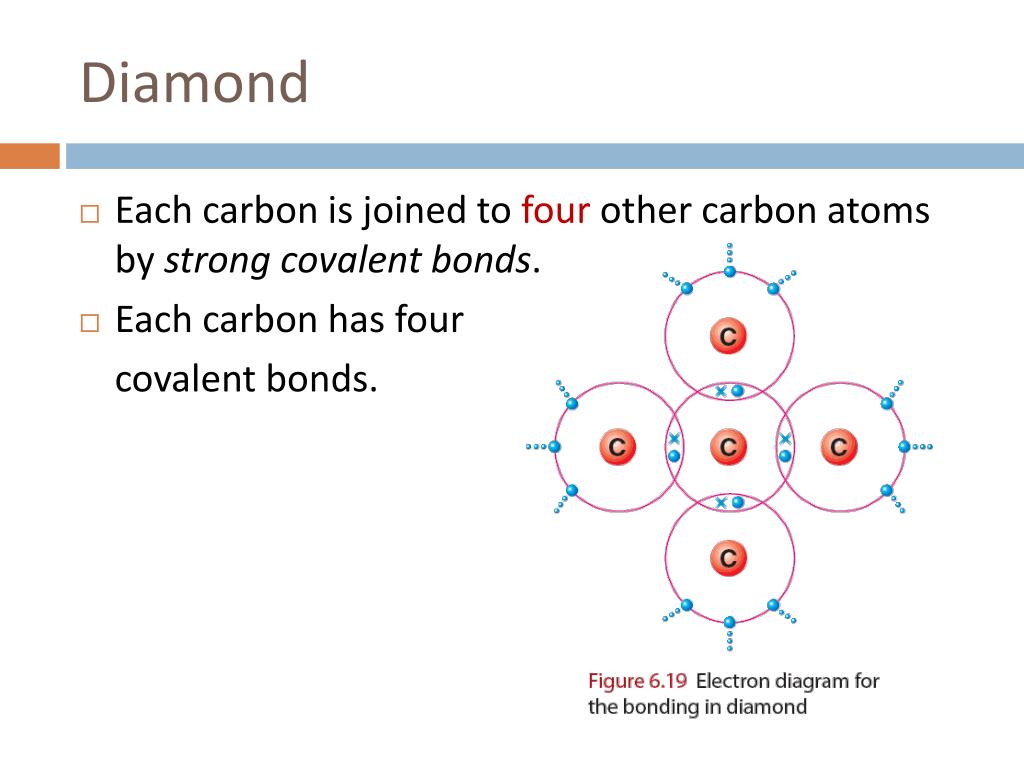
The extraordinary hardness and unique properties of diamond arise from the strong covalent bonds between carbon atoms in its tetrahedral lattice.
Each carbon atom is bonded to four other carbon atoms.
This robust three-dimensional network is responsible for diamond's exceptional strength and resistance to compression.
Addressing the Confusion
The misconception that diamond might be a compound likely stems from a misunderstanding of allotropes and crystalline structures.
Allotropes are different structural forms of the same element. Carbon, for example, exists as diamond, graphite, and fullerenes.
These allotropes have vastly different properties despite being made of the same element.
Another source of confusion might come from the presence of trace impurities within a diamond.
While diamonds can contain small amounts of other elements like nitrogen, these are impurities, not constituent parts of the diamond's fundamental structure.
These trace elements affect the color and other properties, but the core substance remains pure carbon.
Expert Confirmation
“There is absolutely no debate among experts that diamond is an element,” stated Dr. Eleanor Vance, a leading materials scientist at the National Institute of Standards and Technology (NIST).
“It's composed solely of carbon atoms covalently bonded in a distinct crystalline arrangement. The presence of impurities doesn’t change its elemental nature.”
This expert opinion reinforces the consensus within the scientific community.
Furthermore, spectroscopic analysis of diamond consistently reveals the presence of only carbon atoms.
These analyses provide empirical evidence supporting its elemental classification.
Any claims to the contrary are not supported by scientific data.
Implications and Ongoing Clarification
The ongoing confusion highlights the importance of clear and accessible science education.
Educational resources need to emphasize the distinction between elements, compounds, and mixtures.
Simple explanations of allotropes and crystal structures are also crucial.
Several organizations, including the Royal Society of Chemistry and the American Chemical Society, are launching public awareness campaigns to address the misinformation.
These campaigns will use various platforms, including social media and educational videos, to disseminate accurate information about diamond's composition.
Further research into diamond's properties and applications continues to advance our understanding of this remarkable element.
In conclusion, it is critical to reiterate that diamond is an element composed solely of carbon. Efforts are underway to ensure clarity and correct misunderstandings surrounding its fundamental nature.









![Diamond Is A Element Or Compound Is Rust An Element, Compound, or Mixture? [ANSWERED] – Dear Learners](https://dearlearners.com/wp-content/uploads/2021/02/is-diamond-an-element-compound-or-mixture_-768x480.jpg)