Does Zepbound Need To Be Room Temperature Before Use
The rapid rise in popularity of Zepbound, a medication approved for chronic weight management, has led to a surge of questions surrounding its proper usage and storage. Among the most frequently asked: Does Zepbound need to be at room temperature before injection? Misinformation can quickly spread, potentially impacting the drug's efficacy and patient safety.
This article delves into the specific temperature requirements for Zepbound, drawing on official guidelines and expert opinions to provide clarity. Understanding these guidelines is crucial for patients and healthcare providers to ensure the medication's integrity and optimal therapeutic effect.
Understanding Zepbound and its Storage Needs
Zepbound (tirzepatide) is an injectable medication that mimics the effects of certain hormones in the body to regulate appetite and blood sugar levels. It is manufactured by Eli Lilly and is approved for use in conjunction with diet and exercise for chronic weight management in adults.
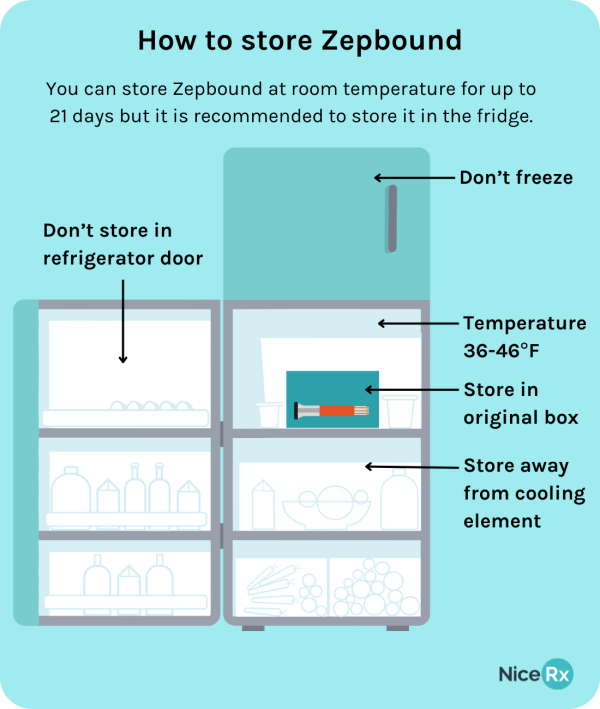
Proper storage is paramount to maintain the drug's stability and effectiveness. According to the official prescribing information, Zepbound should be stored in the refrigerator between 2°C to 8°C (36°F to 46°F).
The medication must be protected from light and should not be frozen. Freezing can damage the medication's structure and render it ineffective.
The Room Temperature Question: Official Guidance
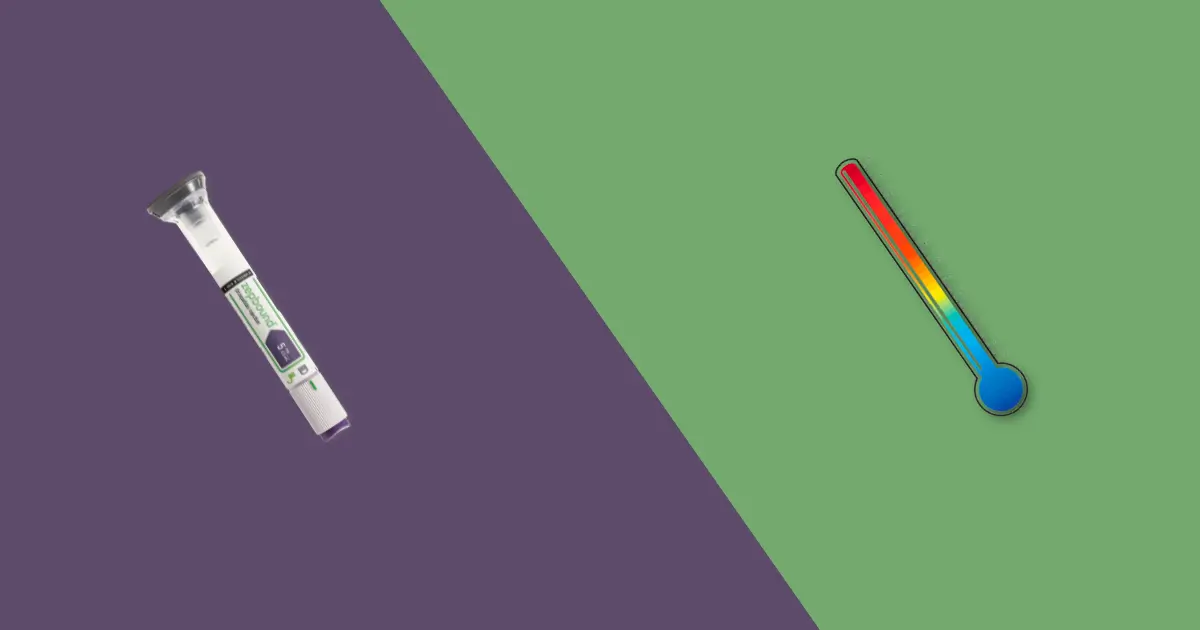
The crucial point of contention lies in whether Zepbound needs to reach room temperature before injection. The prescribing information from Eli Lilly states that if needed, the unrefrigerated, pre-filled pen can be stored at room temperature up to 30°C (86°F) for no more than 21 days.
After this period, it must be discarded, even if it's still within the original expiration date. This indicates that while room temperature storage is permissible for a limited duration, it's not a mandatory step before each injection.
The official guidance does not explicitly instruct users to bring the medication to room temperature immediately before injecting. Instead, it focuses on the overall storage parameters. The assumption is the medication will naturally reach room temperature within a short period after removal from the refrigerator, especially given the small dose volume.
Expert Perspectives and Practical Considerations
While not mandated, some healthcare professionals believe that allowing Zepbound to sit at room temperature for a brief period before injection can enhance patient comfort. Injecting cold medication can sometimes cause localized discomfort or stinging at the injection site.
Dr. Anya Sharma, an endocrinologist specializing in weight management, explains, "Although not required, letting Zepbound warm up slightly for about 15-30 minutes can minimize injection site discomfort for some patients. The active ingredient’s efficacy is not impacted by this slight temperature adjustment, as long as it stays within the overall allowable storage window."
However, the emphasis remains on adhering to the 21-day rule if the pen is kept at room temperature. It's essential to carefully monitor the time if this method is chosen.
Common Misconceptions and Potential Risks
One common misconception is that storing Zepbound at room temperature indefinitely is acceptable. This is dangerous and can significantly degrade the medication's potency.
Another risk involves exposing Zepbound to excessively high temperatures. Exceeding the 30°C (86°F) limit, even temporarily, can compromise the drug's effectiveness. It's crucial to protect the medication from direct sunlight and heat sources.
Using expired or improperly stored Zepbound might result in a reduced therapeutic effect or unexpected side effects. Patients should always inspect the medication for any signs of discoloration or particulate matter before use and consult their healthcare provider if they have any concerns.
Best Practices for Zepbound Usage
To ensure safe and effective use of Zepbound, patients should adhere to the following best practices. Store the pen in the refrigerator between 2°C to 8°C (36°F to 46°F) until ready to use.
If desired, allow the pen to sit at room temperature for a short period (15-30 minutes) before injection. If the pen has been stored at room temperature, ensure it is used within 21 days.
Always follow your healthcare provider's instructions regarding dosage and injection technique. Inspect the medication for any abnormalities before use. Discard the pen properly after each injection.
The Future of Weight Management Medications and Storage Guidelines
As the field of weight management medications continues to evolve, clear and accessible storage guidelines remain vital. Pharmaceutical companies are constantly working to improve the stability and user-friendliness of injectable medications.
Future formulations might offer enhanced temperature stability, potentially reducing the need for strict refrigeration. Educational initiatives aimed at both healthcare professionals and patients are crucial to disseminate accurate information and address any lingering uncertainties surrounding medication storage.
Ultimately, the key to successful Zepbound treatment lies in understanding and adhering to the official storage guidelines and consulting with healthcare providers for personalized guidance. Staying informed will allow patients to maximize the medication's benefits while minimizing potential risks.