Energetics Of Hybridization Wikipedia
Imagine a world where the building blocks of molecules dance to a tune dictated by energy itself. Atoms, eager to find stability, contort and reshape their electronic neighborhoods. This isn't science fiction, but a peek into the heart of chemical bonding, a concept brought to life on platforms like Wikipedia through the exploration of hybridization.
At its core, the energetics of hybridization, as detailed on the Wikipedia page, describes how atomic orbitals – the regions around an atom where electrons are likely to be found – mix to form new, hybrid orbitals with different shapes and energies. This process isn't just a mathematical exercise; it's a fundamental principle that explains the geometry and reactivity of countless molecules.
The Dance of Atomic Orbitals
Before diving into the specifics, it's important to understand the cast of characters. Atomic orbitals, often denoted as s, p, d, and f, each possess a unique spatial distribution. These orbitals represent the probability of finding an electron in a given region of space around an atom's nucleus.
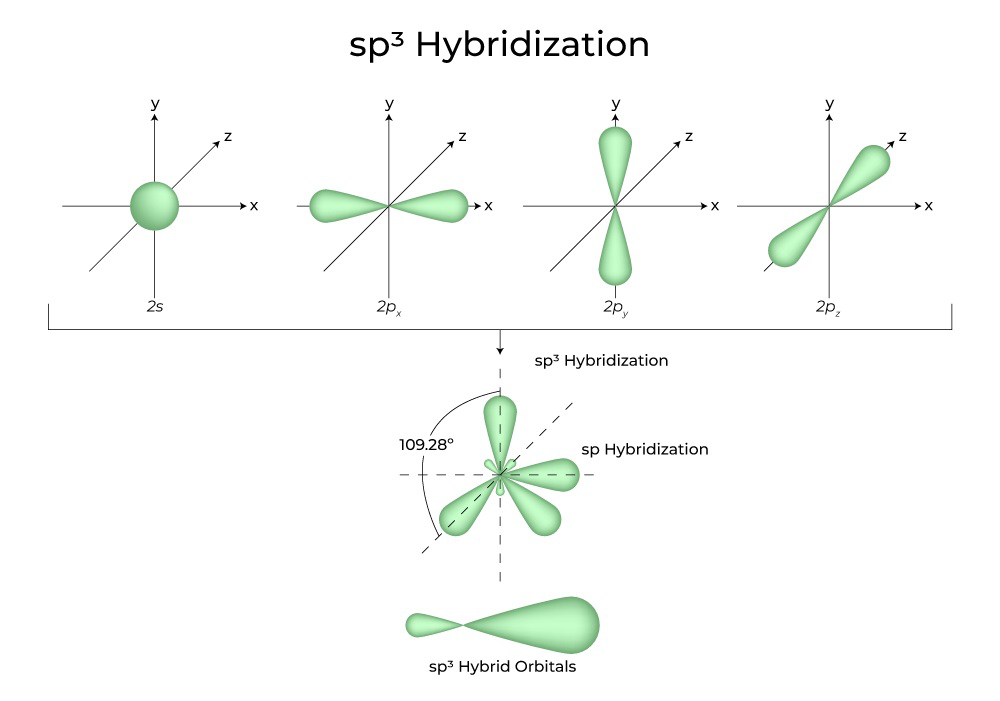
Hybridization occurs when these atomic orbitals combine to create new orbitals. These newly formed hybrid orbitals are specifically suited for bonding. This blending allows atoms to form stronger, more stable bonds with other atoms.
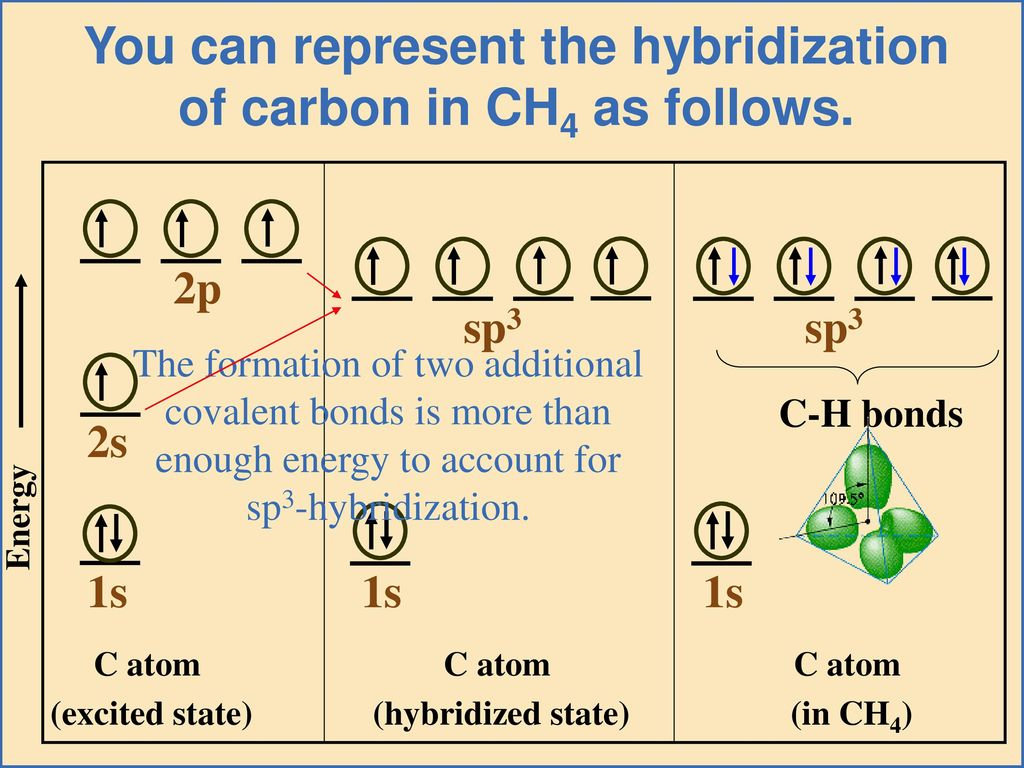
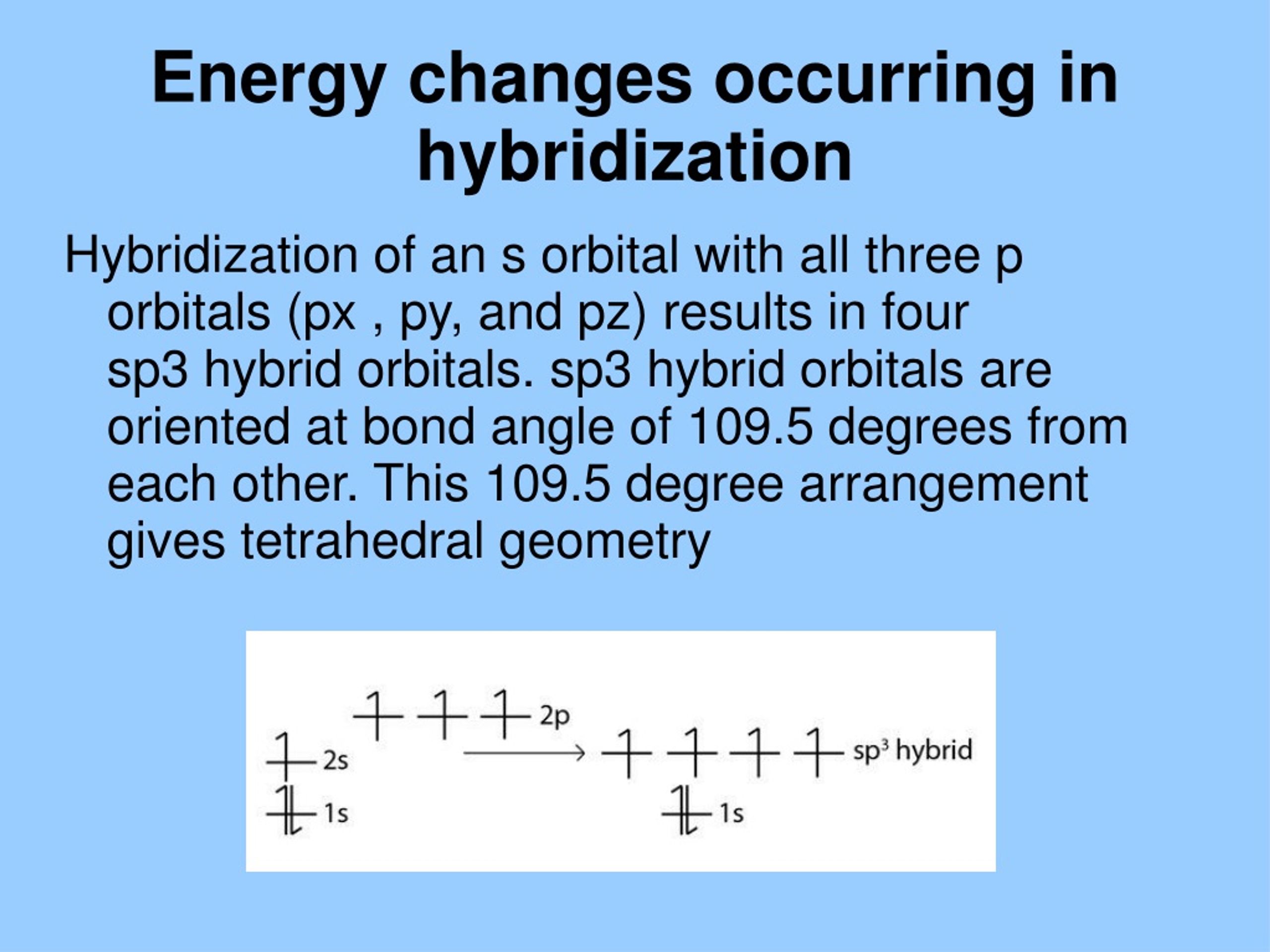
The concept of hybridization was first proposed by Linus Pauling in the 1930s. He needed to explain the observed tetrahedral geometry of methane (CH4). His groundbreaking work bridged quantum mechanics and observed molecular structures.
Types of Hybridization: A Spectrum of Bonds
The Wikipedia page diligently outlines the different types of hybridization. Each corresponds to different molecular geometries and bonding properties.
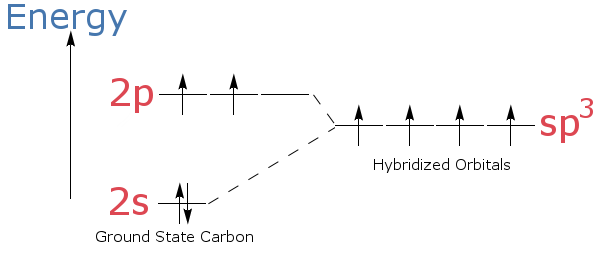
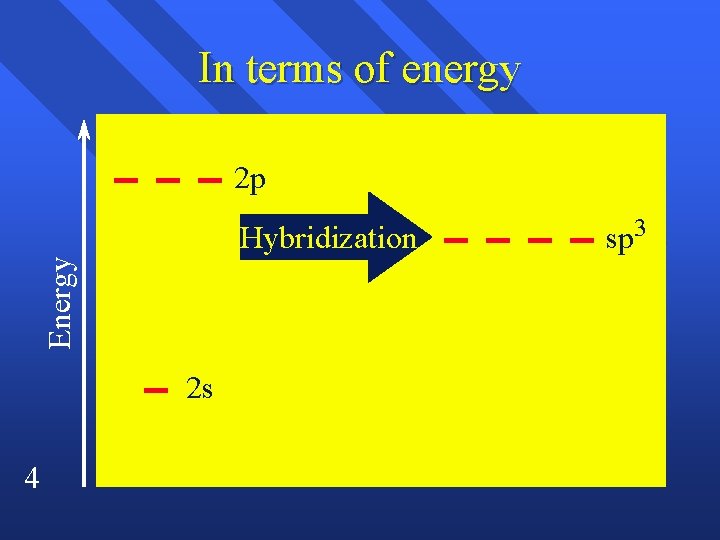
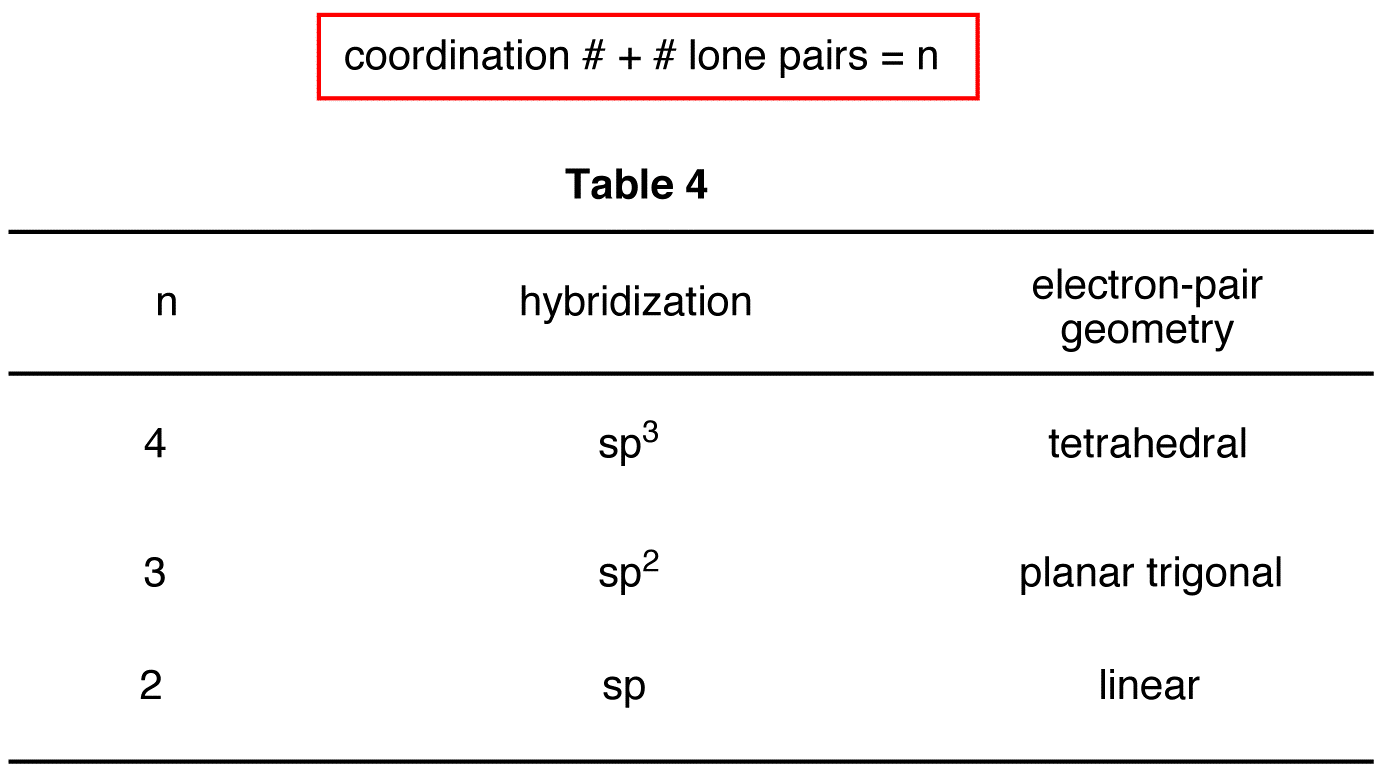
sp3 hybridization, for instance, involves mixing one s orbital and three p orbitals to create four equivalent hybrid orbitals. These orbitals point towards the corners of a tetrahedron, explaining the structure of methane and many other organic molecules.
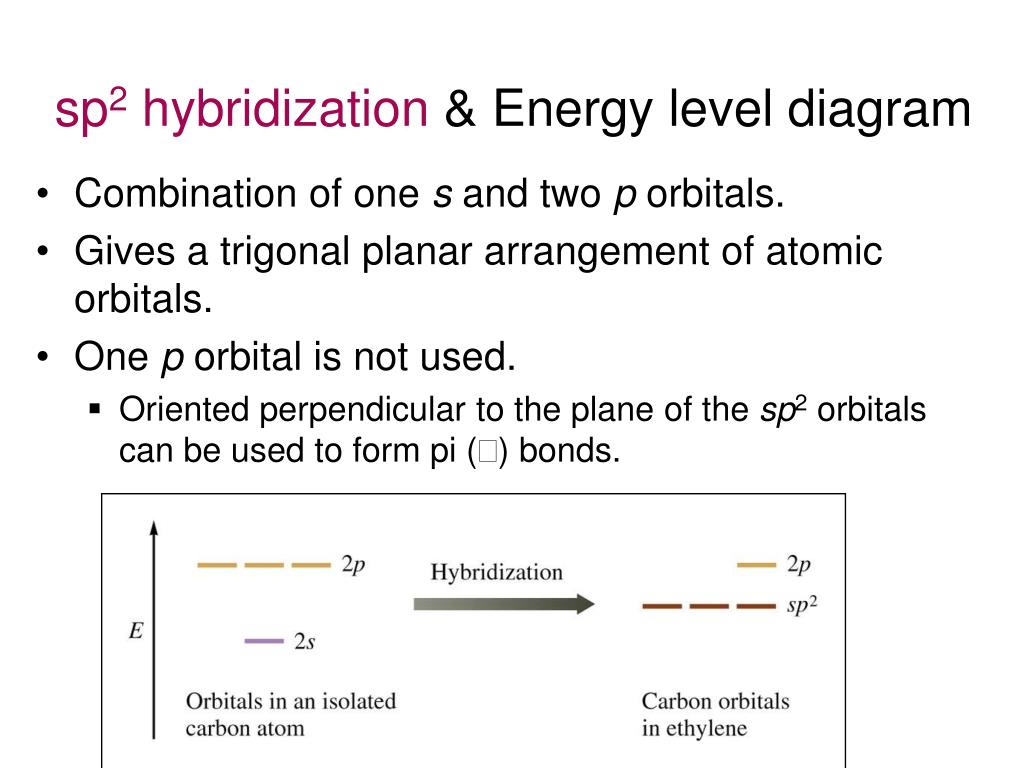
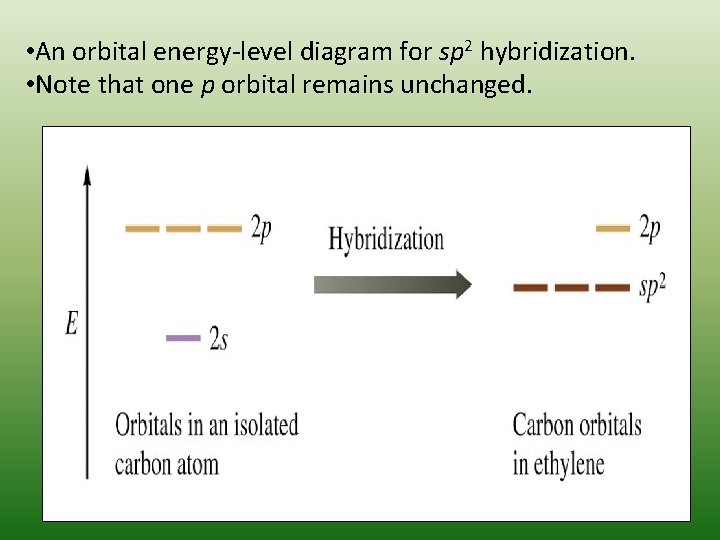
Then there’s sp2 hybridization, where one s orbital combines with two p orbitals. The result are three hybrid orbitals arranged in a trigonal planar geometry, with one unhybridized p orbital remaining. This type of hybridization is found in molecules like ethene (C2H4), where the unhybridized p orbital forms a π bond, in addition to the σ bonds formed by the sp2 orbitals.
sp hybridization involves the mixing of one s orbital and one p orbital. This creates two hybrid orbitals arranged linearly, with two unhybridized p orbitals. Acetylene (C2H2), with its triple bond, exemplifies this type of hybridization.
Energetics: The Driving Force
The process of hybridization isn't spontaneous; it requires energy input. The overall energetic outcome, however, is usually favorable. The energy released during bond formation with the newly formed hybrid orbitals outweighs the energy needed for hybridization.
This energy balance is crucial in determining whether a molecule will adopt a particular geometry. The energetics of hybridization are often discussed using simplified energy level diagrams on Wikipedia.
These diagrams visually represent the relative energies of atomic and hybrid orbitals, showcasing the energy "cost" of hybridization. They also highlight the "benefit" of forming stronger bonds.
Significance and Applications
The understanding of hybridization has revolutionized the fields of chemistry and materials science. It provides a framework for predicting and explaining molecular shapes, bond strengths, and reactivity.
Knowledge about hybridization is vital when designing new molecules with specific properties. Molecular engineers rely on hybridization principles to create new pharmaceuticals, polymers, and catalysts.
Furthermore, hybridization is crucial in understanding the properties of various materials. For example, the strength and flexibility of carbon-based materials, like diamond and graphite, are directly related to the hybridization states of carbon atoms.
Wikipedia: A Gateway to Understanding
The Wikipedia page on energetics of hybridization serves as a valuable resource for students and researchers alike. It offers a comprehensive overview of the topic.
However, Wikipedia's collaborative nature means that its content evolves. It requires users to exercise critical thinking. It is important to cross-reference the information with other reliable sources.
Ultimately, the energetic principles that drive hybridization are a testament to the beauty and complexity of the molecular world. Wikipedia offers a accessible way to explore these concepts.
The ability of atoms to adapt and reshape their electronic configurations underscores the dynamic nature of chemistry. It highlights the constant quest for stability that underlies all matter.
.jpg)





.jpg?revision=1&size=bestfit&width=502&height=160)