Evaluate The Contract Research Organization For Medical Devices Company Avania

Imagine a bustling lab, researchers huddled over complex machinery, their faces illuminated by the soft glow of monitors displaying intricate data. The air hums with the quiet intensity of scientific discovery, each experiment a step closer to bringing a life-changing medical device to market. But behind the scenes, often unseen, lies a crucial partner: the Contract Research Organization (CRO).
This article delves into the role of CROs, specifically focusing on Avania, a company making waves in the medical device sector. We'll explore what sets them apart and examine their impact on the journey from concept to commercialization, considering both their strengths and areas where the landscape presents challenges.
The Vital Role of CROs in Medical Device Development
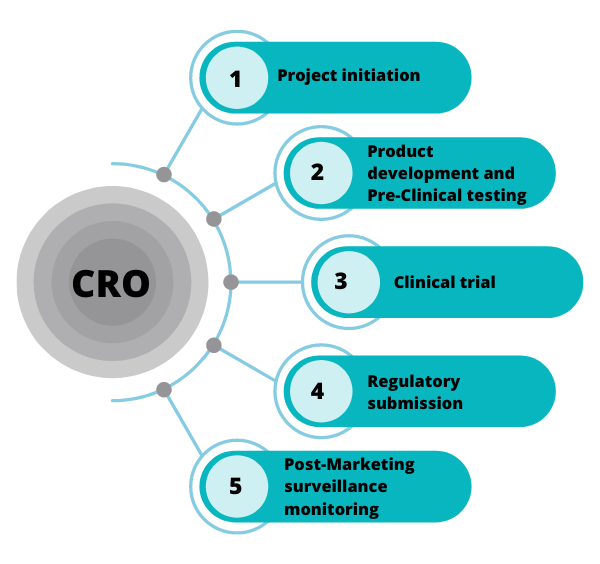
Developing a medical device is a complex, costly, and highly regulated process. Companies, especially smaller startups, often lack the in-house expertise and resources to navigate the intricate maze of preclinical testing, clinical trials, regulatory submissions, and post-market surveillance.
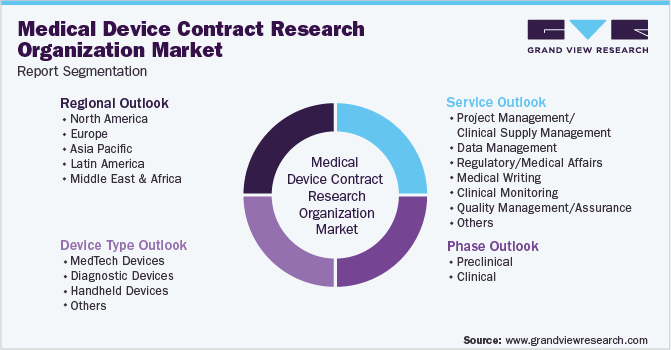
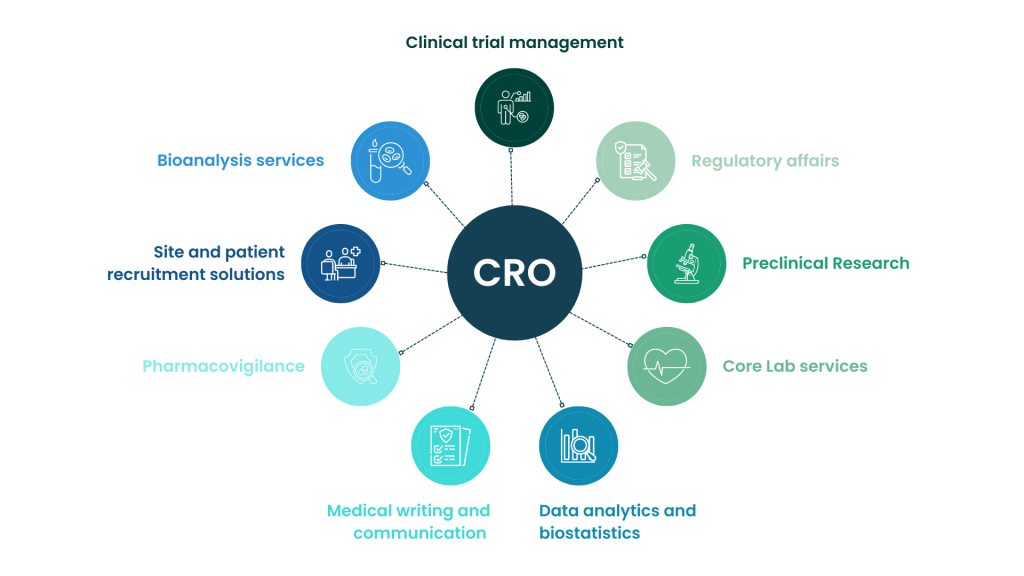
That’s where CROs step in. These organizations provide specialized support, acting as an extension of the medical device company’s team. They offer a range of services, from study design and data management to site management and regulatory consulting.
Avania: A Closer Look
Avania is a global CRO exclusively focused on medical devices and diagnostics. Their singular focus differentiates them from larger, more diversified CROs that also handle pharmaceuticals. This specialization, they argue, allows them to offer a deeper understanding of the unique challenges and opportunities within the medical device industry.
Founded with a mission to accelerate medical device innovation, Avania emphasizes a collaborative approach, working closely with its clients to tailor solutions to their specific needs. Their website highlights their expertise in various therapeutic areas, including cardiovascular, orthopedic, and neurological devices.
Strengths of Avania
Several factors contribute to Avania's reputation as a leading CRO in the medical device space. One key advantage is their deep regulatory expertise. Navigating the FDA (in the US), the EMA (in Europe), and other international regulatory bodies requires a thorough understanding of complex guidelines and standards.
Avania boasts a team of regulatory specialists who can guide companies through the submission process, helping to ensure compliance and minimize delays. This expertise is particularly valuable for startups entering the market for the first time.
Another strength is their focus on clinical trial management. From site selection and patient recruitment to data collection and analysis, Avania provides comprehensive support throughout the clinical trial process. They utilize advanced technologies and data management systems to ensure data integrity and efficiency.
Their experience with a wide range of medical devices, from Class I to Class III, allows them to tailor their approach to the specific requirements of each project. This adaptability is crucial in an industry characterized by constant innovation and technological advancements.
Furthermore, Avania emphasizes building strong relationships with their clients. This collaborative approach fosters open communication and transparency, allowing for proactive problem-solving and efficient project management. Clients often praise their responsiveness and dedication to achieving shared goals.
Considerations and Potential Challenges
While Avania presents a compelling value proposition, it's important to acknowledge potential challenges and considerations. One factor is cost. CRO services can be a significant investment for medical device companies, particularly early-stage ventures with limited budgets.
It's essential to carefully evaluate the costs and benefits of outsourcing to a CRO versus building an in-house team. A thorough cost-benefit analysis can help companies make informed decisions about their resource allocation.
Another consideration is the potential for communication breakdowns. While Avania emphasizes collaboration, maintaining effective communication between the medical device company and the CRO team is crucial. Clear communication protocols and regular updates are essential to ensure that everyone is aligned on project goals and timelines.
Additionally, choosing the right CRO requires careful due diligence. It’s important to research the CRO’s experience, expertise, and track record in the specific therapeutic area and device class. Seeking references from previous clients and conducting thorough interviews can help companies assess the CRO’s suitability for their needs.
The Impact of CROs on Medical Device Innovation
CROs like Avania play a significant role in accelerating medical device innovation. By providing specialized expertise and resources, they enable companies to focus on their core competencies – developing innovative technologies and improving patient care.
They help to streamline the regulatory process, conduct efficient clinical trials, and gather high-quality data. This not only reduces the time and cost of bringing new devices to market but also increases the likelihood of regulatory approval and commercial success.
In a rapidly evolving healthcare landscape, the ability to quickly and efficiently develop and commercialize medical devices is more important than ever. CROs are instrumental in helping companies navigate this complex environment and deliver innovative solutions to patients in need.
"The medical device industry is constantly evolving, and CROs must adapt to meet the changing needs of their clients," says a recent industry report by the Medical Device Manufacturers Association (MDMA).
Looking Ahead
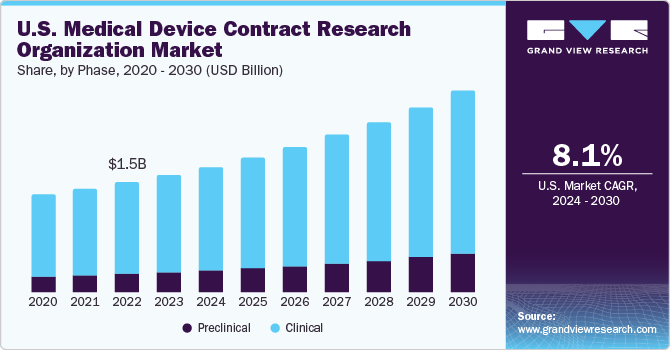
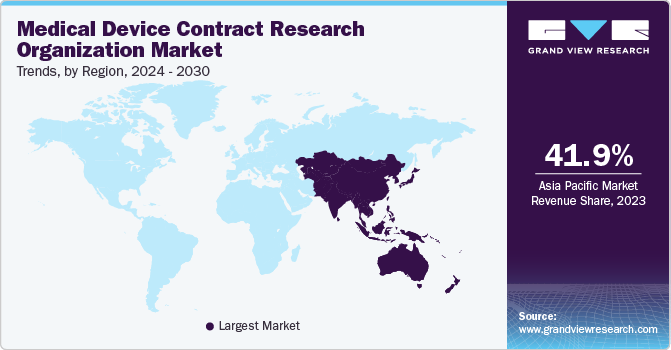
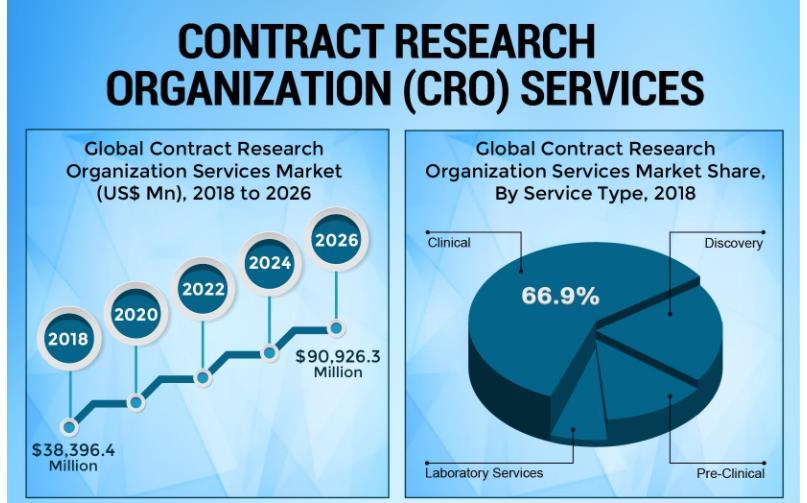
The demand for CRO services in the medical device industry is expected to continue to grow in the coming years. As regulatory requirements become more stringent and the pace of innovation accelerates, companies will increasingly rely on CROs to support their development and commercialization efforts.
Companies like Avania are well-positioned to capitalize on this trend, leveraging their expertise, experience, and collaborative approach to help medical device companies bring innovative solutions to market. Their continued success will depend on their ability to adapt to the changing needs of the industry and maintain their commitment to quality and innovation.
Ultimately, the partnership between medical device companies and CROs is a critical component of the healthcare ecosystem. By working together, they can drive innovation, improve patient outcomes, and shape the future of medical technology.







-1.png)




