How Many Electrons Are In Nickel
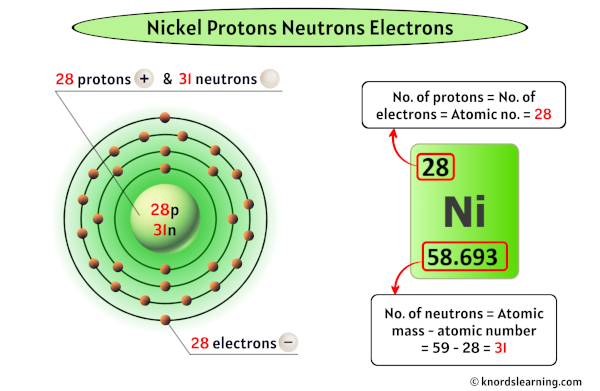
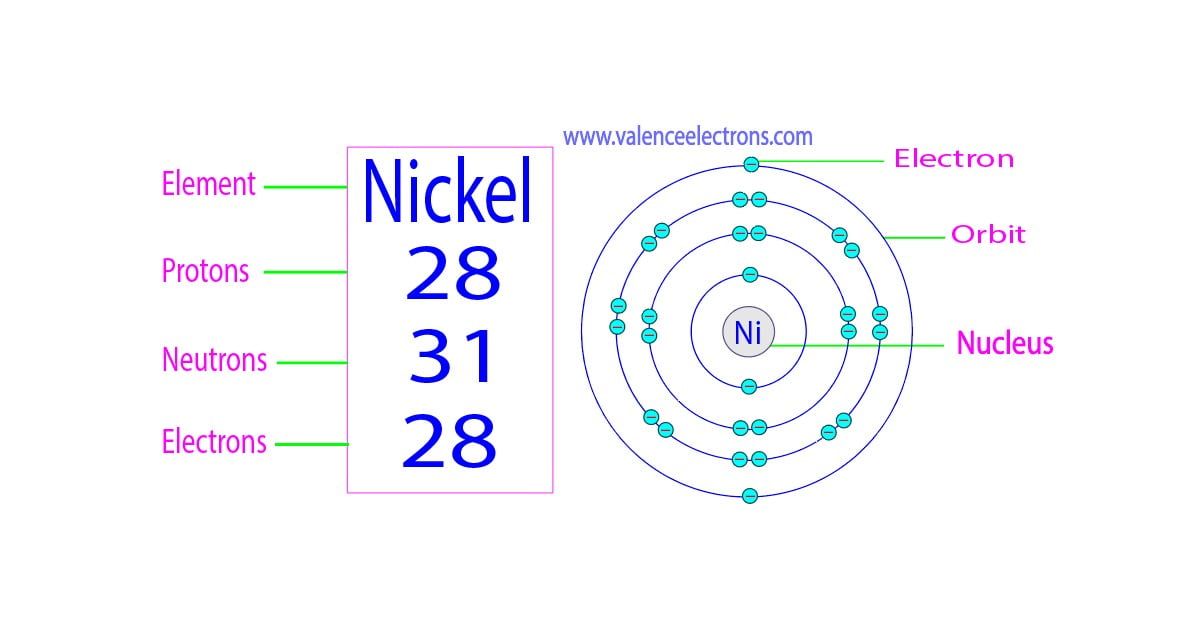
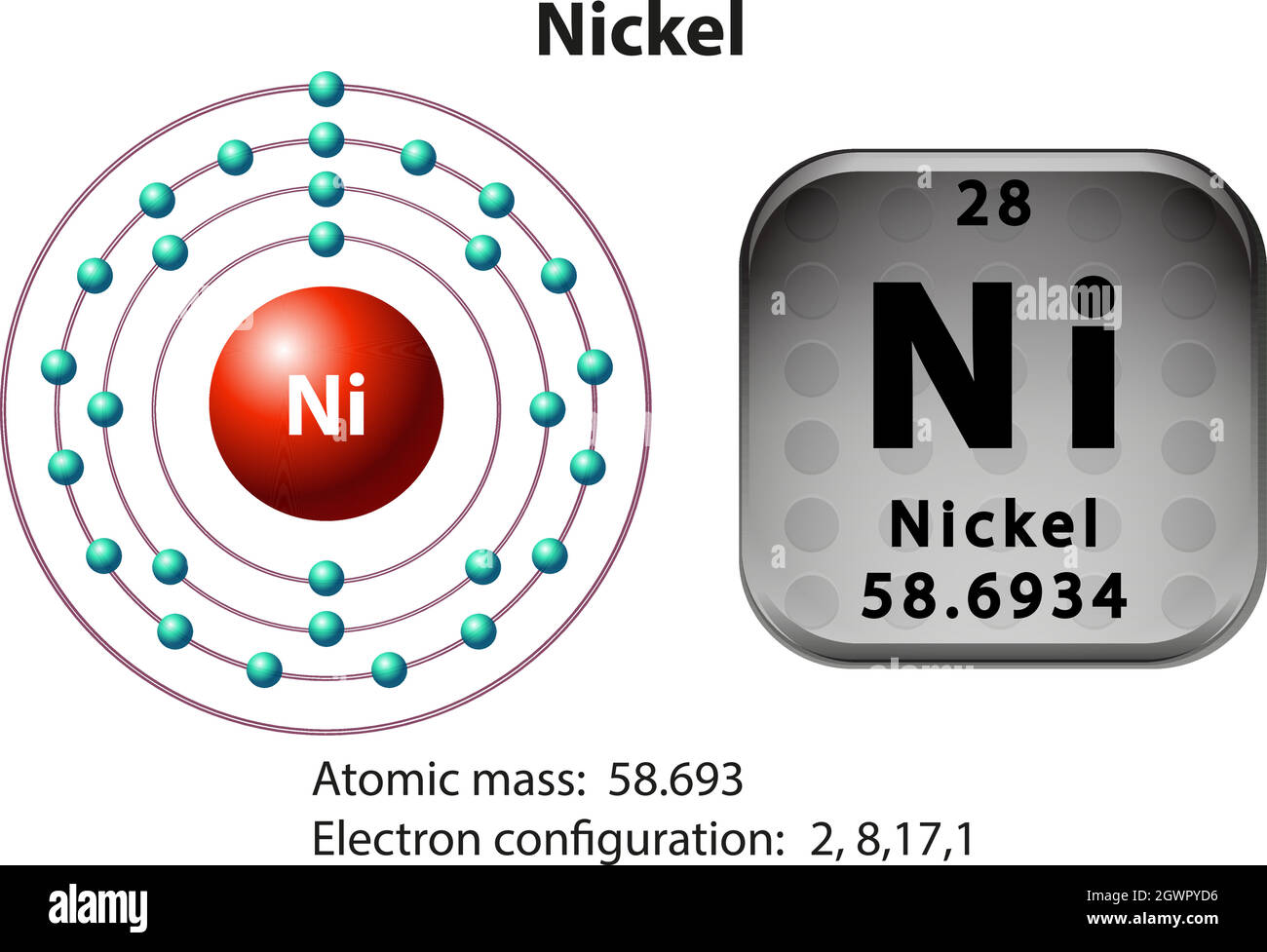
Immediate confirmation is in: Nickel atoms contain 28 electrons. This number dictates its chemical behavior and place on the periodic table.
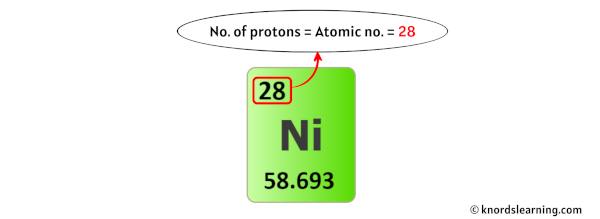
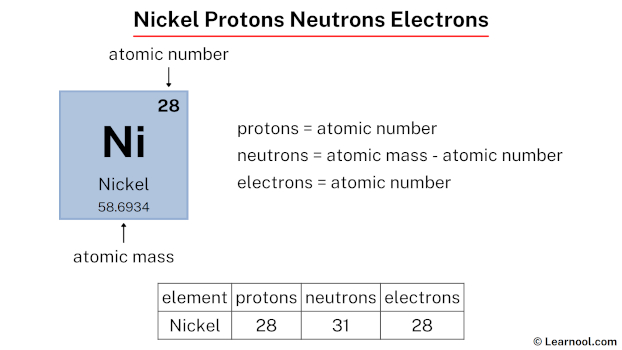
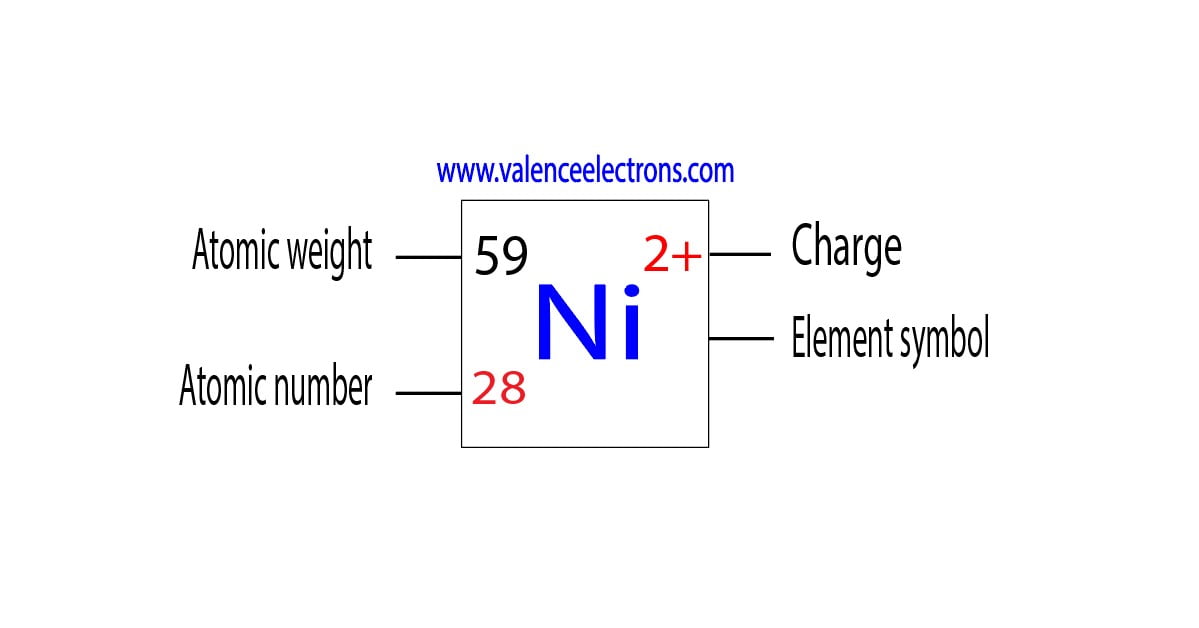
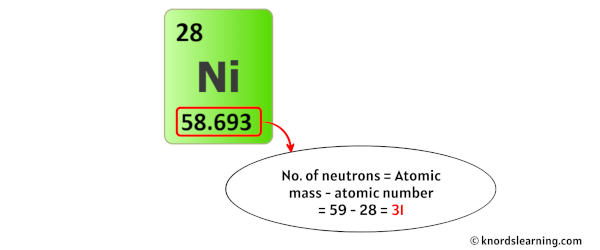
The atomic number of nickel, 28, directly corresponds to the number of protons in its nucleus and, in a neutral atom, the number of electrons orbiting the nucleus. This crucial information underpins all of nickel's properties and interactions.
Nickel's Electron Count: A Deep Dive
Who: The element in question is nickel, symbolized as Ni.
What: We are determining the number of electrons in a neutral atom of nickel.
Where: This information is universally applicable, regardless of nickel's location.
When: The number of electrons in a neutral nickel atom remains constant, unless ionized.
How: The number of electrons is derived directly from nickel's atomic number on the periodic table.
A neutral atom of any element must have an equal number of positively charged protons and negatively charged electrons.
Therefore, since nickel has 28 protons, it must also have 28 electrons to maintain electrical neutrality.
Electron Configuration
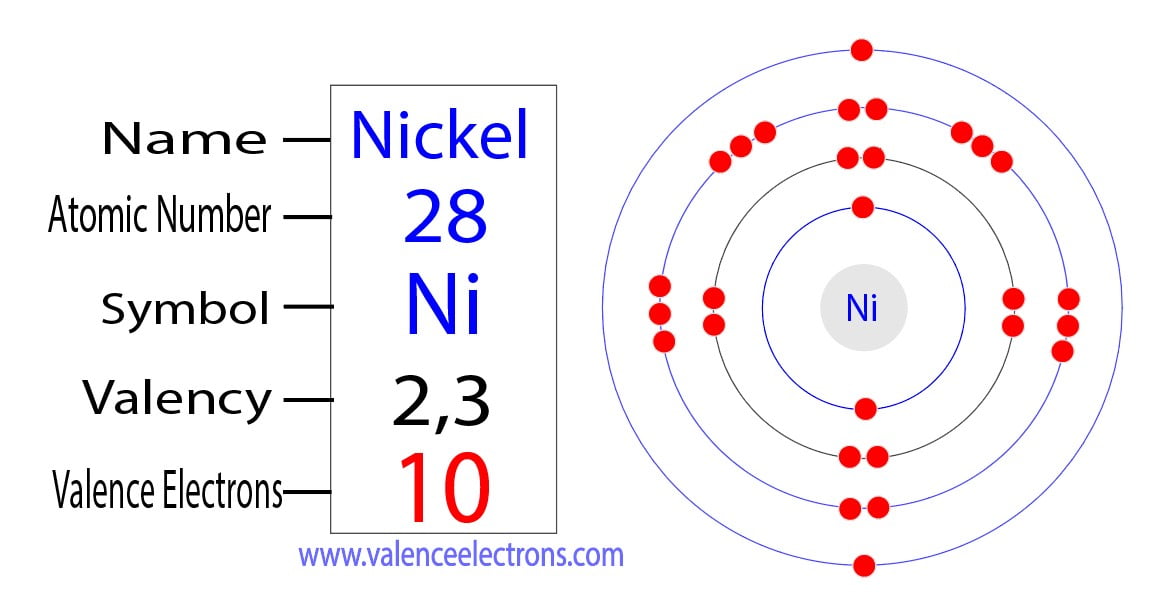
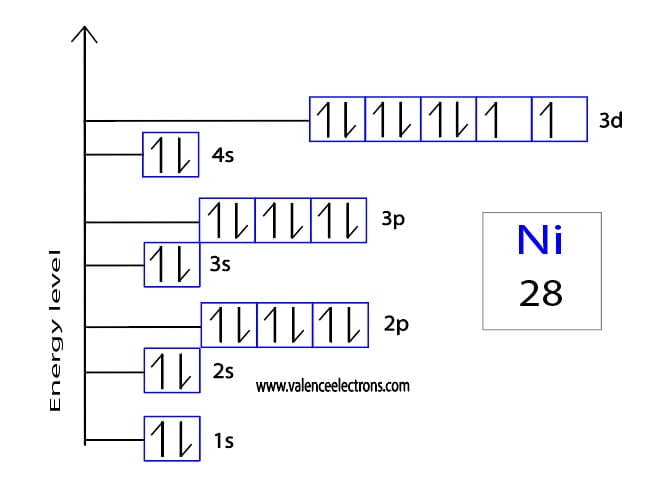
Nickel's electron configuration is [Ar] 3d8 4s2.
This configuration signifies how the 28 electrons are arranged within the atom's energy levels and orbitals.
Understanding this configuration is vital for predicting nickel's bonding behavior and chemical reactivity.
The 3d orbitals are particularly important, as they are involved in many of nickel's chemical reactions and contribute to its metallic properties.
Nickel Ions and Electron Changes
Nickel can lose or gain electrons to form ions.
For example, Ni2+ has lost two electrons and possesses only 26 electrons.
These ionic forms are critical in various chemical compounds and biological systems.
The most common oxidation state for nickel is +2.
Understanding the number of electrons in these ions is essential for comprehending their chemical behavior.
Significance of Electron Count
The 28 electrons in nickel dictate its chemical properties, including its ability to form strong bonds.
Nickel is a transition metal known for its catalytic activity.
Its electron configuration allows it to readily form complexes with other elements and molecules, facilitating various chemical reactions.
Nickel is used in many industrial processes, often as a catalyst.
Knowing the precise number of electrons is paramount for modeling these reactions and developing new technologies.
Furthermore, understanding nickel's electron structure is crucial for predicting its interactions in various materials, such as alloys and electronic devices.
Conclusion
The confirmed electron count for a neutral nickel atom is 28. Ongoing research will further refine our understanding of how these electrons influence nickel's behavior at the atomic and molecular level.








![How Many Electrons Are In Nickel Nickel (Ni) - Periodic Table [Element Information & More]](https://knordslearning.com/wp-content/uploads/2023/01/nickel-element-periodic-table.jpg)