Which Elements Are Metals Check All That Apply

Imagine strolling through a sun-drenched museum, each exhibit gleaming under the soft light. You pause, captivated by a display showcasing a periodic table brought to life – vibrant chunks of copper, shimmering bars of gold, and sturdy blocks of iron. It’s a reminder of the elements that construct our world, elements that often share surprising common characteristics, yet are distinctly individual.
This article dives into the fascinating world of metals, exploring which elements on the periodic table qualify for this classification. Understanding which elements are metals is essential to grasping chemistry, material science, and countless technologies that shape our daily lives.
Metals make up a significant portion of the periodic table, but determining their precise boundaries requires a nuanced understanding of their properties.
The Defining Characteristics of Metals
Metals aren't just about that shiny appearance; they possess a set of key properties that distinguish them from nonmetals and metalloids. These properties define their behavior and applications.
Conductivity: The Flow of Electrons
One of the most defining characteristics of metals is their excellent conductivity of both electricity and heat. This stems from their atomic structure, where electrons can move freely, allowing them to easily carry electrical charge or thermal energy.
Think of copper wires in your home or the aluminum that helps cool your laptop – these wouldn't work without this free flow of electrons.
Luster: The Metallic Shine
Metals often have a distinctive shine or luster. This results from their ability to reflect light effectively, creating that characteristic gleam we associate with metals like silver and gold.
This reflective property makes them valuable in jewelry, mirrors, and decorative applications.
Malleability and Ductility: Shaping Metals
Malleability and ductility describe a metal's ability to be shaped without breaking. Malleability refers to the ability to be hammered into thin sheets, while ductility describes the ability to be drawn into wires.
Iron, for example, can be forged into various shapes, and copper can be drawn into fine wires used in electronics.
Other Distinguishing Properties
Other important metal properties include their typically high melting and boiling points. They tend to be solid at room temperature, with exceptions like mercury.
Metals also tend to lose electrons to form positive ions (cations).
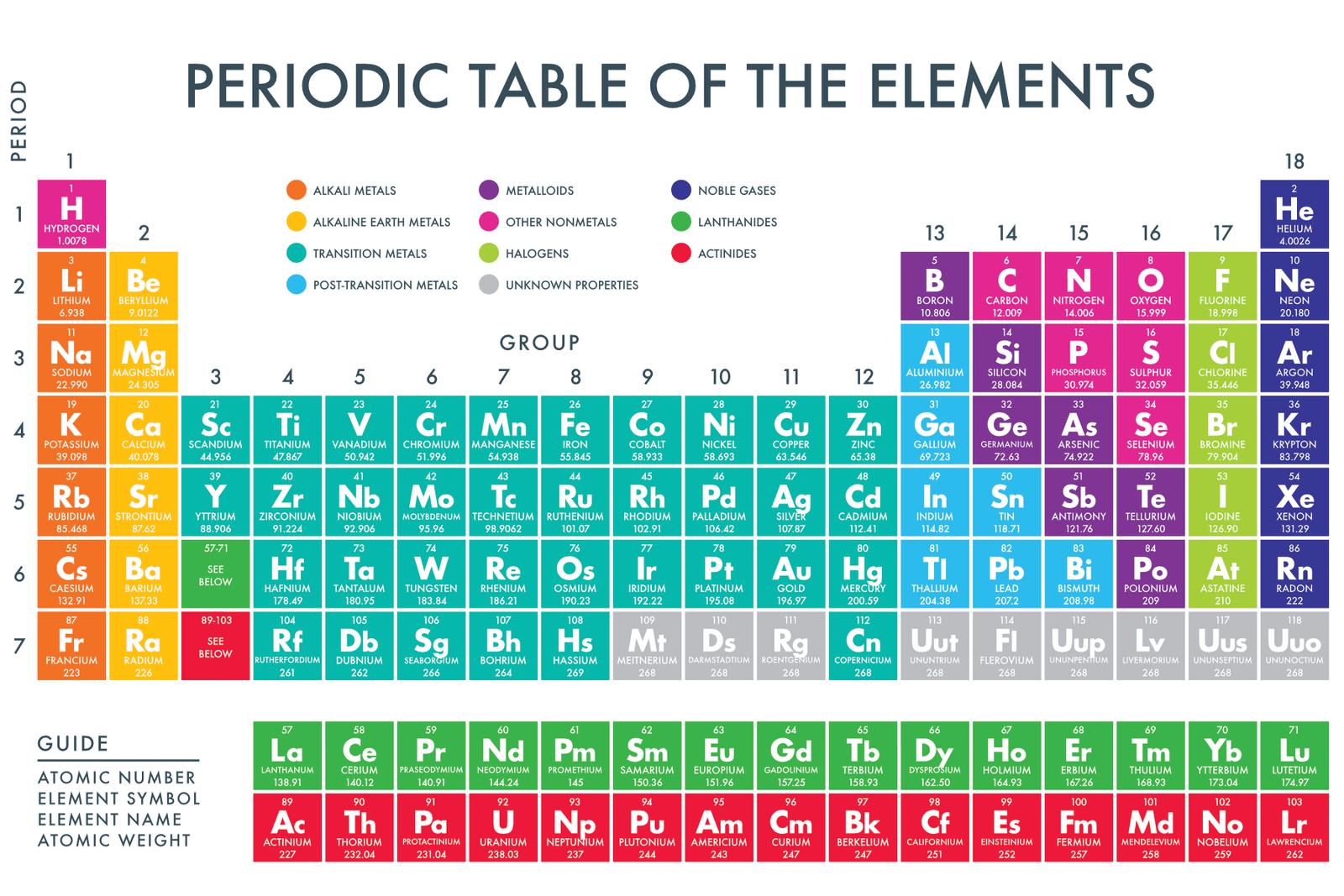
Exploring the Metallic Elements
Now, let's look at some of the prominent metallic elements on the periodic table. We'll focus on different groups to illustrate the range of metallic behavior.
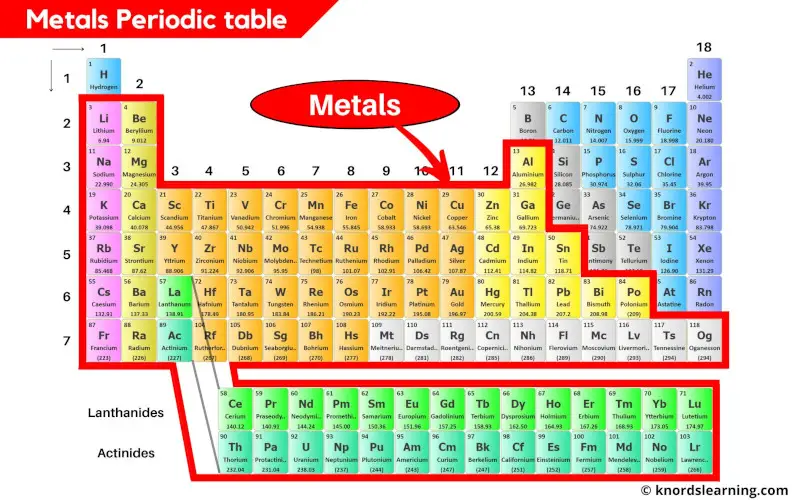
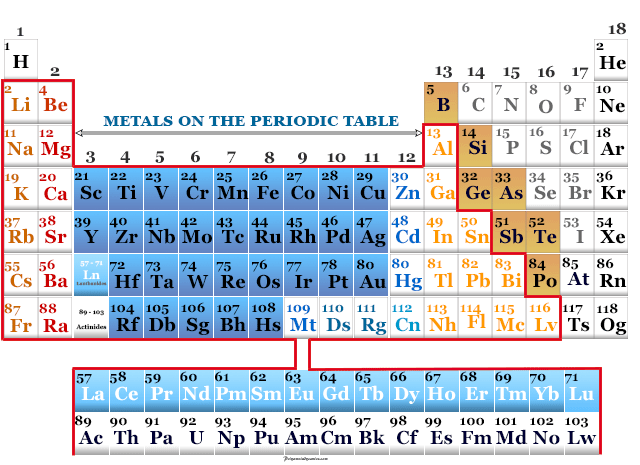
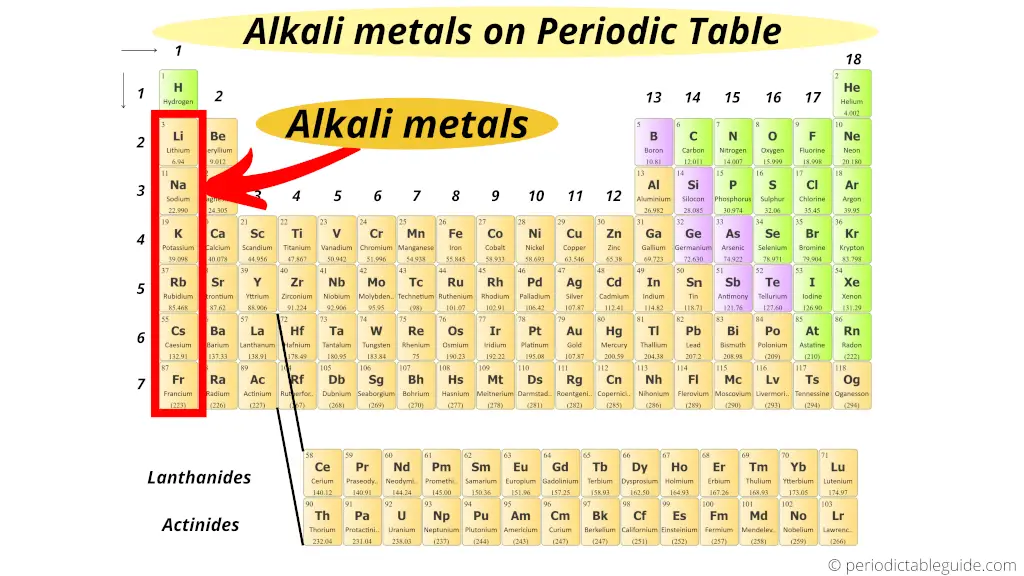
Alkali Metals: Group 1
The alkali metals, including lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr), are highly reactive metals. They readily lose one electron to form positive ions.
Their reactivity increases as you move down the group, with francium being the most reactive, though it's also very rare and radioactive. These metals react violently with water.
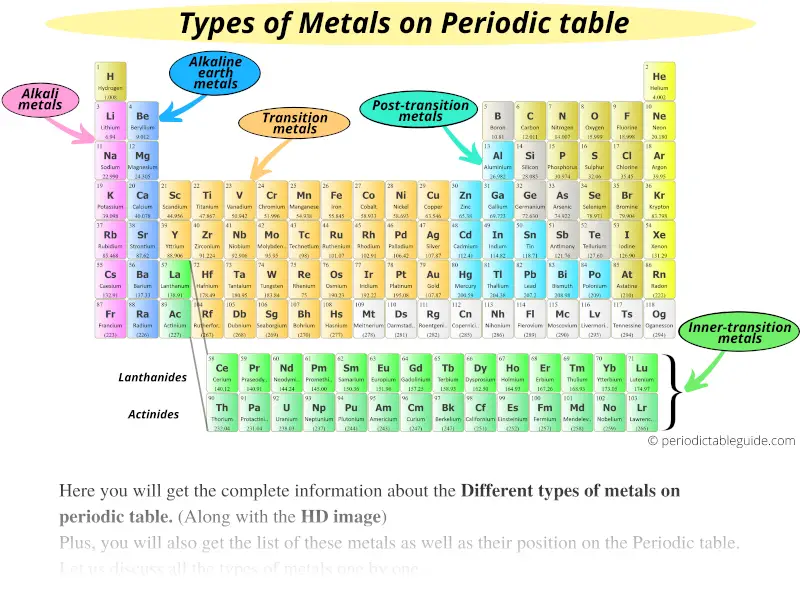
Alkaline Earth Metals: Group 2
The alkaline earth metals, beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra) are also reactive, but less so than alkali metals. They tend to lose two electrons to form positive ions.
Magnesium is used in lightweight alloys, and calcium is essential for bone health.
Transition Metals: The Heart of Metallicity
The transition metals occupy the central block of the periodic table. They are characterized by variable oxidation states and often form colorful compounds.
This group includes familiar metals like iron (Fe), copper (Cu), gold (Au), silver (Ag), and titanium (Ti), all with distinct properties and applications.
Transition metals are known for their strength, hardness, and use as catalysts.
Lanthanides and Actinides: The Inner Transition Metals
These two series of elements, often displayed below the main body of the periodic table, are also metals. Lanthanides, like cerium (Ce) and neodymium (Nd), are often used in alloys and magnets.
Actinides, including uranium (U) and plutonium (Pu), are radioactive and primarily used in nuclear applications.
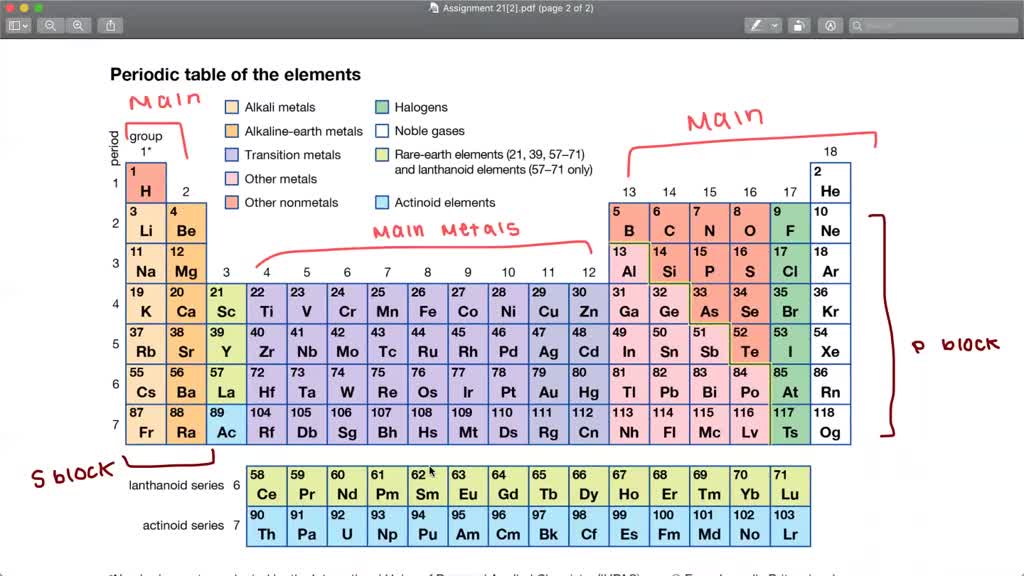
Basic Metals: The P-Block Metals
Located in the p-block, these metals include aluminum (Al), gallium (Ga), indium (In), tin (Sn), thallium (Tl), lead (Pb), and bismuth (Bi). Their metallic character is generally weaker compared to transition metals.
Aluminum is lightweight and corrosion-resistant, making it ideal for aerospace and packaging. Lead, though toxic, was historically used in plumbing and paints.
The Gray Area: Metalloids and the Metallic Boundary
It's important to acknowledge that the boundary between metals and nonmetals isn't always clear-cut. Elements known as metalloids (or semi-metals) possess properties intermediate between those of metals and nonmetals.
Metalloids include boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), and polonium (Po). Their semiconducting properties make them essential in electronics. Silicon is the backbone of the computer industry.
Determining whether an element is a metal requires considering multiple properties, not just one. Some elements might exhibit metallic luster but poor conductivity, blurring the lines.
Why Does It Matter? The Significance of Metallic Elements
The identification and understanding of metallic elements are crucial for many reasons. They play a fundamental role in various fields, from industry to biology.
Many industries rely on the unique properties of metals. Construction utilizes steel (an alloy of iron), electronics depend on copper and gold, and transportation relies on aluminum and titanium.
These metals provide strength, conductivity, and durability.
Certain metals are also essential for biological processes. Iron is a component of hemoglobin, which carries oxygen in the blood. Zinc is vital for immune function.
These metals help keep us alive and thriving.
Moreover, the discovery and manipulation of metals have driven technological advancements throughout history. From the Bronze Age to the Iron Age to the modern era, metals have shaped tools, weapons, and infrastructure.
According to the U.S. Geological Survey (USGS), the demand for certain critical metals, such as lithium and cobalt, is rapidly increasing due to their use in batteries for electric vehicles and energy storage. Securing stable and sustainable sources of these metals is a critical challenge.
The study of metals also extends to understanding their potential environmental impacts. Mining and processing of metals can lead to pollution, and proper management is essential to minimize harm.
In Conclusion: A World Built on Metals
As we conclude our journey through the realm of metallic elements, it's clear that they are much more than just shiny, conductive materials. They are the building blocks of our technology, the lifeblood of many biological processes, and the cornerstone of countless industries.
The diverse properties of metallic elements and their crucial role in shaping our world make them a topic of continuous research and fascination. From the strong foundations of skyscrapers to the delicate circuits of microchips, metals shape the world around us.
Understanding which elements are metals empowers us to appreciate their significance and to utilize them responsibly for the benefit of society.