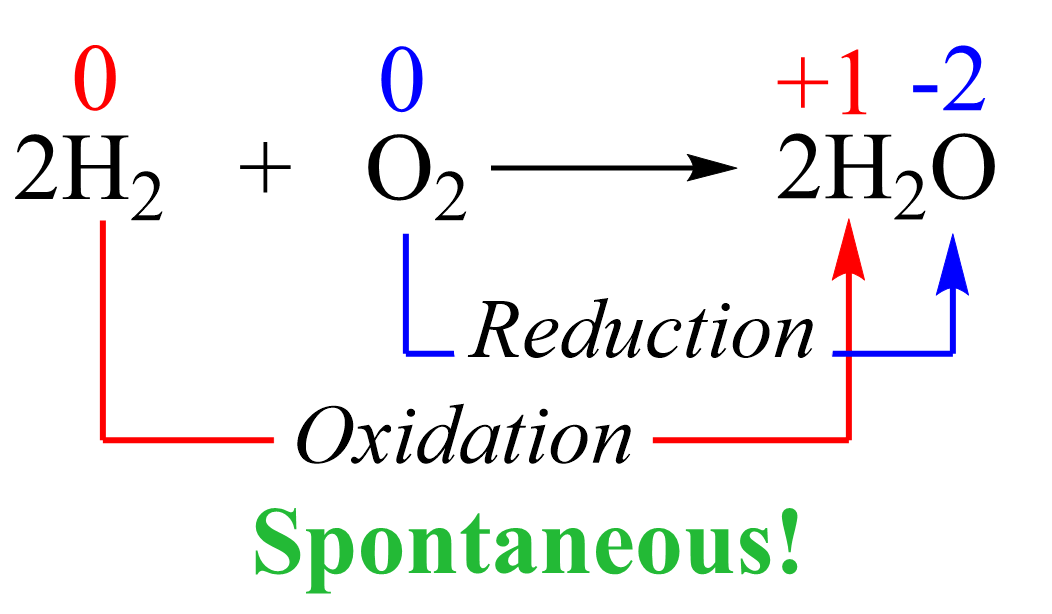How To Add Hydrogen To Water

Urgent research breakthroughs have simplified hydrogen infusion into water. New methodologies promise accessible and efficient hydrogen-rich water production.
This article details validated methods for adding hydrogen to water, bypassing complex laboratory setups and focusing on techniques suitable for wider adoption. The information provided focuses on direct, replicable methods and eschews speculative or unverified claims.
Electrolysis: A Proven Method
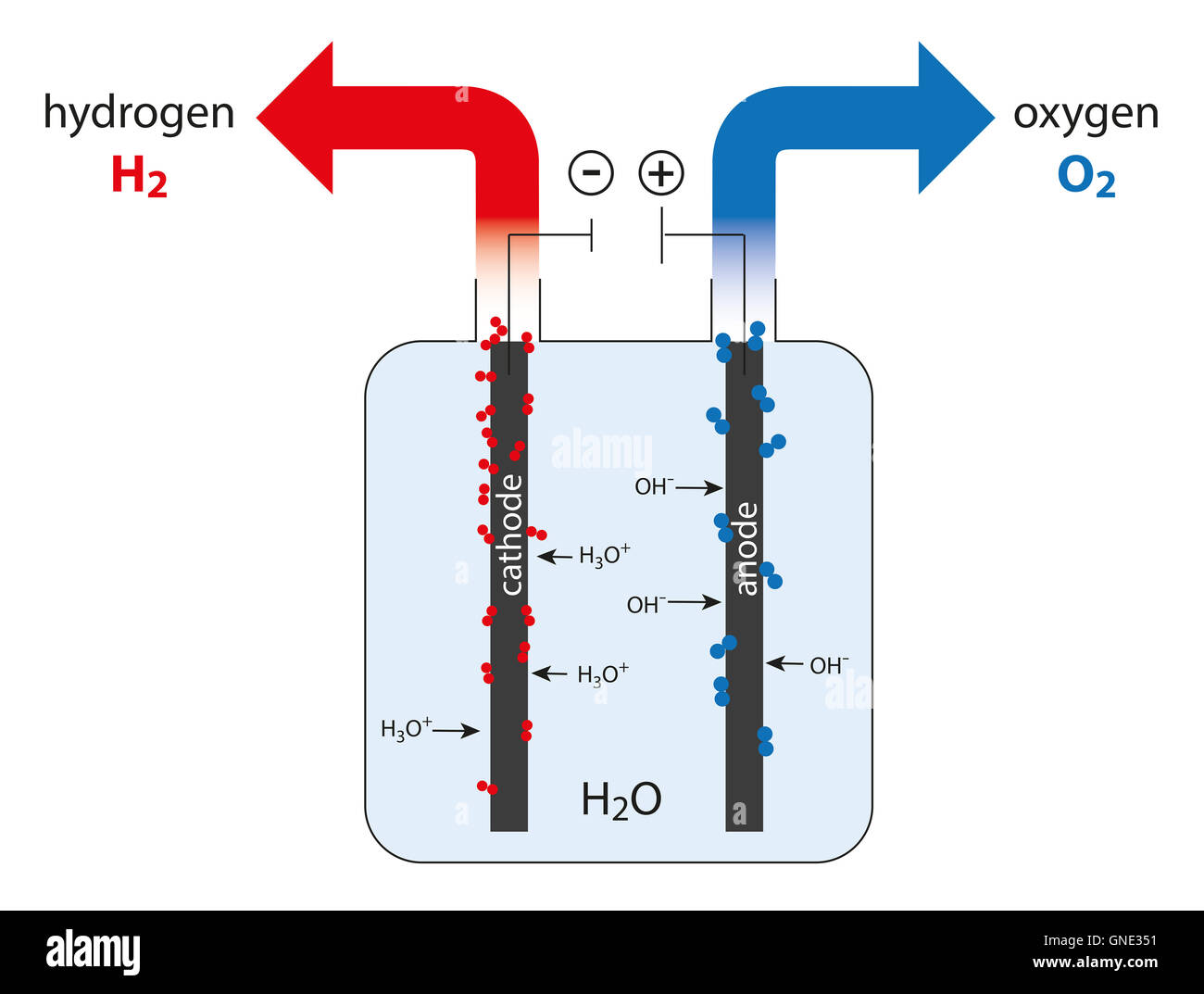
Electrolysis is a well-established method for separating water (H2O) into its constituent elements: hydrogen (H2) and oxygen (O2). This process involves passing an electric current through the water.
WHO: Anyone with access to an electrolyzer and distilled water. WHAT: Splitting water into hydrogen and oxygen.
HOW: An electrolyzer, consisting of an anode (positive electrode) and a cathode (negative electrode) immersed in water, requires a direct current (DC) power source. Water alone is a poor conductor, so adding an electrolyte (like potassium hydroxide, KOH) improves conductivity.
When current flows, hydrogen gas forms at the cathode, and oxygen gas forms at the anode. The gases can then be collected separately.
Essential Considerations for Electrolysis
The purity of the water is paramount. Use only distilled or deionized water to prevent contamination and ensure efficient hydrogen production.
Electrolyte type and concentration influence efficiency. Research and select a suitable electrolyte based on your electrolyzer design.
Electrolyzer design impacts hydrogen purity. Systems with membranes separating the electrodes prevent gas mixing.
Magnesium Reaction: A Simpler Approach
A more direct method involves the reaction of magnesium (Mg) with water. This reaction produces hydrogen gas and magnesium hydroxide (Mg(OH)2).
WHO: Individuals familiar with basic chemistry and safety protocols. WHAT: Reacting magnesium with water to produce hydrogen. WHERE: Well-ventilated areas due to hydrogen's flammability.
HOW: Place magnesium metal (e.g., magnesium ribbon or powder) into water. The reaction is slow at room temperature, however, the rate can be accelerated by using hot water or adding a small amount of acid (like hydrochloric acid, HCl) as a catalyst.
The chemical reaction is: Mg(s) + 2 H2O(l) → Mg(OH)2(aq) + H2(g). WHEN: Reaction begins immediately upon contact between magnesium and water.
Safety Precautions for Magnesium Reaction
Hydrogen is highly flammable. Perform this reaction in a well-ventilated area away from open flames or sparks.
The reaction can generate heat. Use caution and appropriate containers.
Dispose of magnesium hydroxide properly. It is a mildly alkaline substance.
Hydrogen Gas Infusion: Direct Dissolution
Another method involves directly infusing hydrogen gas into water. This requires a source of pure hydrogen gas and a suitable gas infusion system.
WHO: Researchers or commercial entities with access to hydrogen gas. WHAT: Dissolving hydrogen gas into water.
HOW: Bubble hydrogen gas through water using a diffuser. To increase hydrogen concentration, use pressurized systems or smaller bubbles to maximize the surface area of contact between the gas and the water.
Temperature affects hydrogen solubility. Colder water holds more dissolved hydrogen.
Optimizing Hydrogen Gas Infusion
Use high-purity hydrogen gas to avoid contamination of the water. Impurities in the gas can affect the quality of the hydrogen-rich water.
Pressurized systems improve hydrogen dissolution. Henry's Law states that the solubility of a gas in a liquid is directly proportional to the partial pressure of that gas above the liquid.
Measure dissolved hydrogen concentration with appropriate sensors. Ensure accurate monitoring of hydrogen levels.
Nanobubble Technology: An Emerging Field
Emerging research explores using nanobubble technology to enhance hydrogen dissolution. Nanobubbles are extremely small gas bubbles that remain stable in liquid for extended periods.
WHO: Advanced research laboratories. WHAT: Creating stable nanobubbles of hydrogen in water.
HOW: Specialized equipment generates nanobubbles of hydrogen. These nanobubbles increase the surface area for hydrogen dissolution, leading to higher concentrations.
The long-term stability and effectiveness of nanobubble technology are still under investigation. More research is needed to validate its practical applications.
While promising, nanobubble technology remains complex and less accessible compared to electrolysis and magnesium reaction methods.
Moving Forward
Ongoing research aims to optimize these methods for efficiency and scalability. Improving electrolyzer designs, exploring new catalysts for magnesium reaction, and refining nanobubble technology are active areas of development.
Continuous advancements promise more accessible and cost-effective ways to produce hydrogen-rich water. Keep abreast of the latest scientific publications for updates and validated methods.
Further research will dictate the prevalence and adoption of each methods, but the outlined techniques remain the most confirmed ways to produce hydrogen-rich water today.