How To Increase The Concentration Of A Solution

In laboratories and industries worldwide, the ability to precisely control the concentration of solutions is paramount. From pharmaceutical formulations to environmental monitoring, achieving the desired concentration is often critical for accurate results and successful outcomes.
Understanding and mastering the techniques for increasing the concentration of a solution is a fundamental skill, requiring careful consideration of the solute, solvent, and intended application.
The Science Behind Solution Concentration
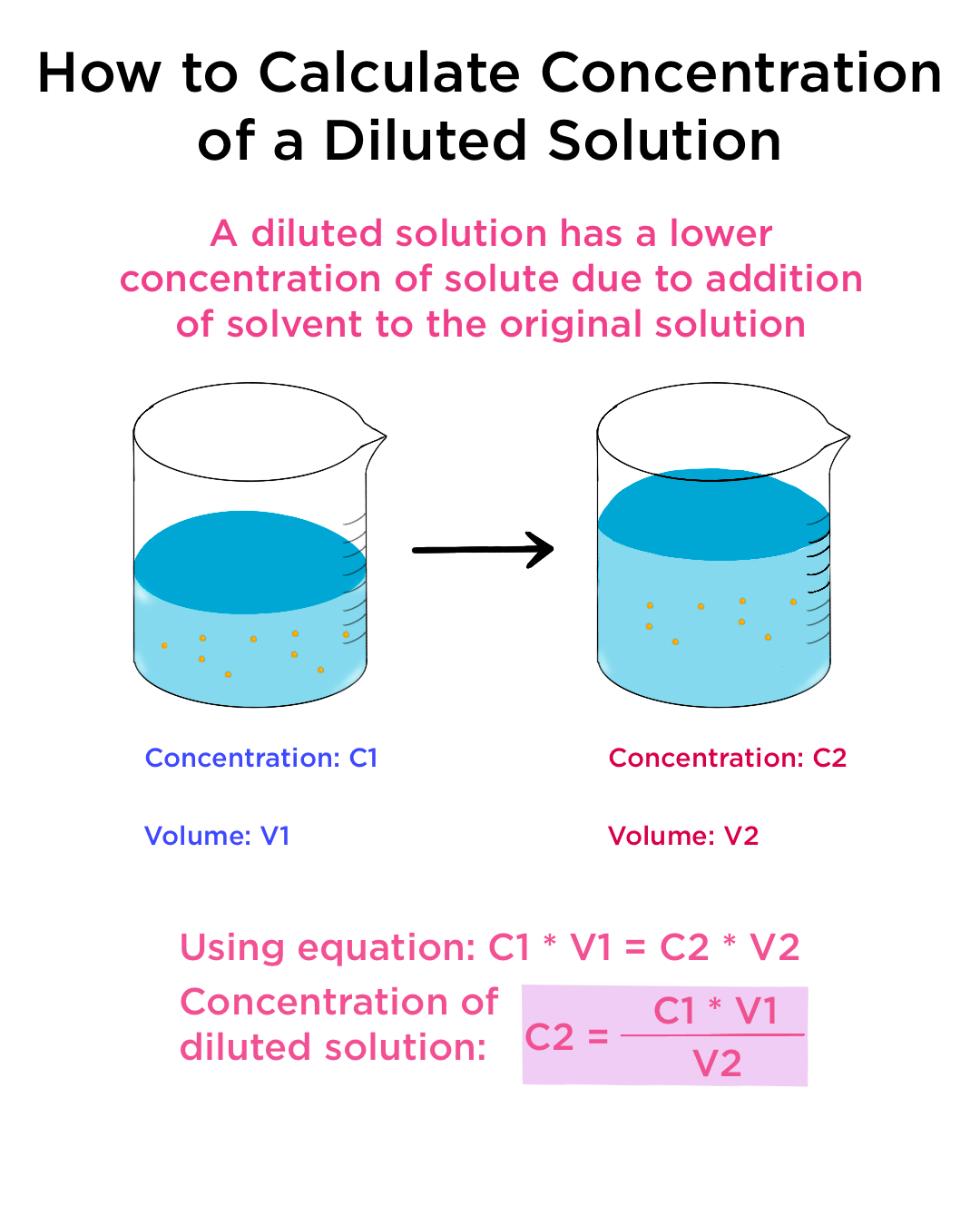
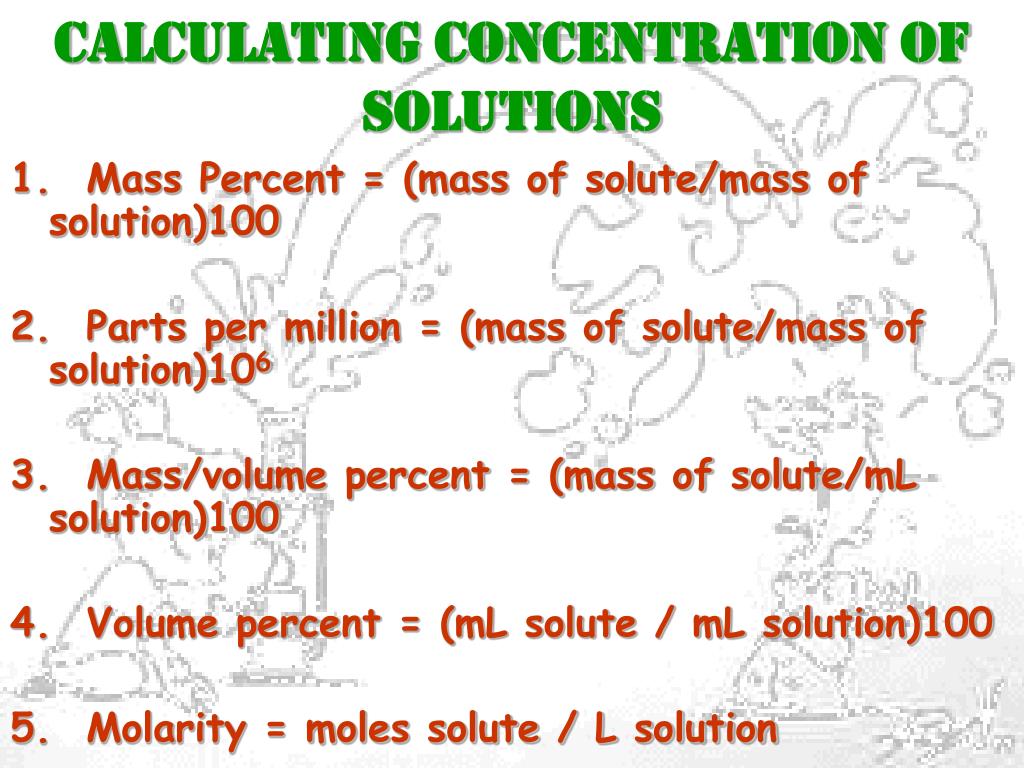
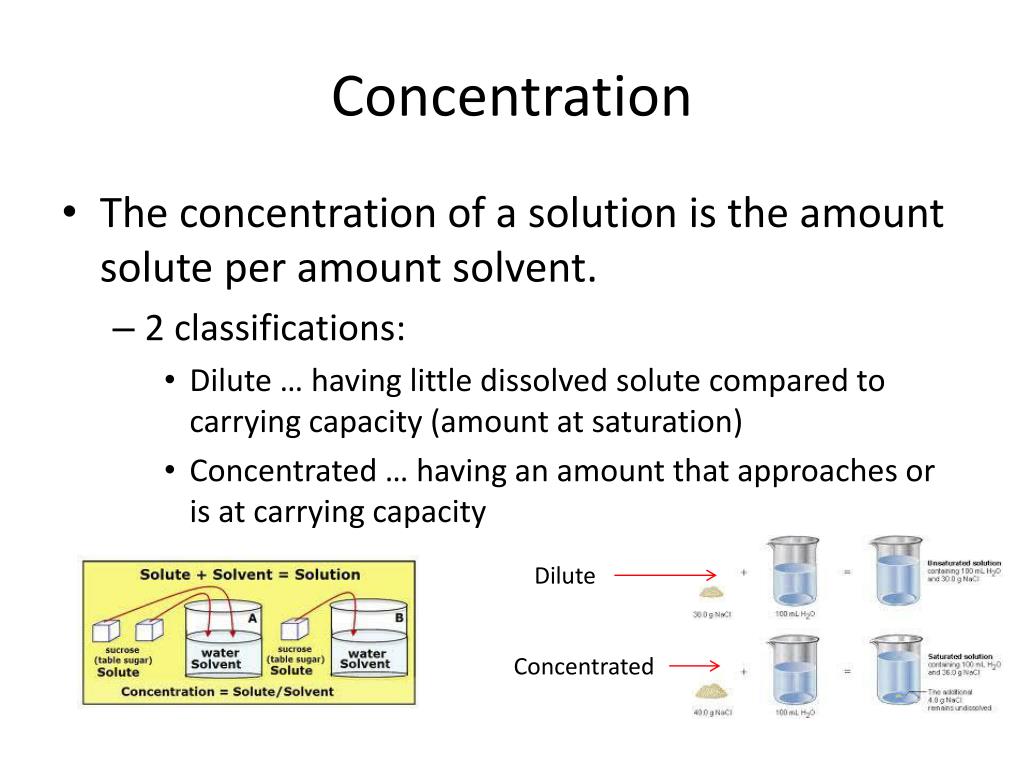
At its core, concentration refers to the amount of solute present in a given amount of solvent or solution. It's a quantitative measure that reflects the relative proportions of these components.
Increasing concentration essentially means increasing the proportion of solute relative to the solvent. Various methods exist to achieve this, each suited to specific types of solutions and applications.
Methods for Increasing Solution Concentration
Evaporation: A Tried-and-True Technique
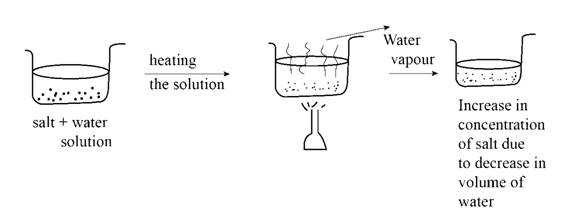
Evaporation is perhaps the most common and straightforward method for increasing concentration. This process involves selectively removing the solvent, typically through heating, leaving behind a solution with a higher proportion of solute.
The efficiency of evaporation depends on factors such as temperature, surface area, and the vapor pressure of the solvent. In industrial settings, specialized evaporators are often used to accelerate the process and minimize solvent loss.
However, evaporation can be problematic if the solute is volatile or heat-sensitive. Dr. Anya Sharma, a leading chemist at the National Institute of Standards and Technology (NIST), cautions that "excessive heating can lead to decomposition of the solute, rendering the solution unusable."
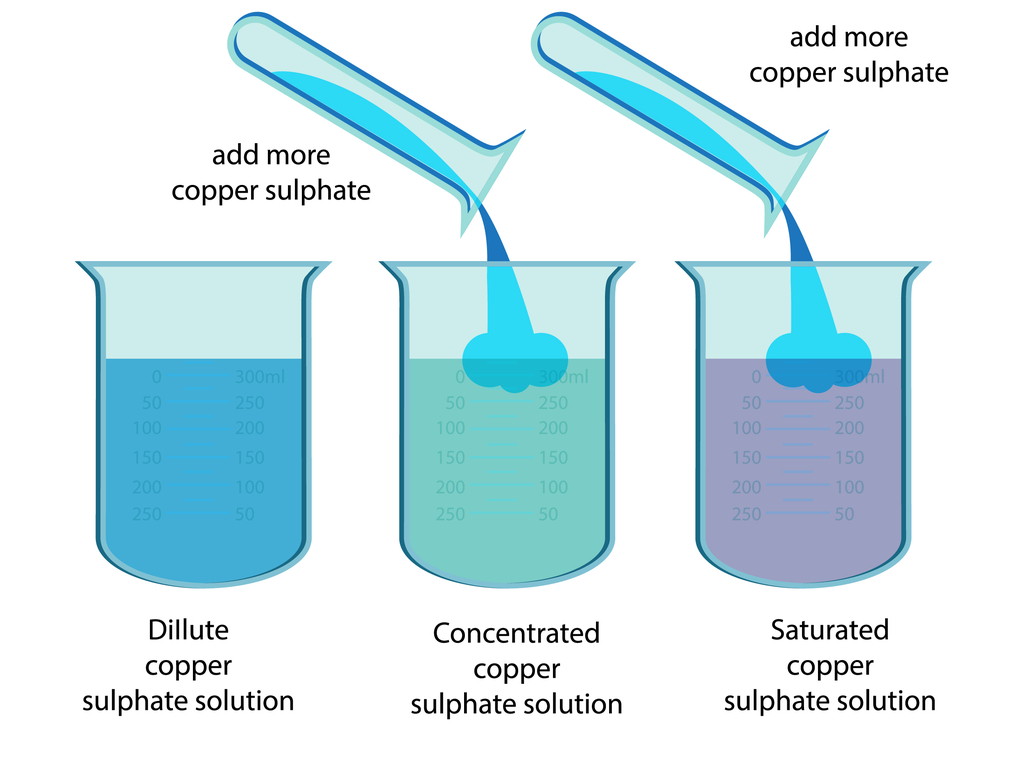
Adding More Solute: A Simple Approach
Another method for increasing concentration is simply adding more solute to the existing solution. This is particularly effective when the solute is readily soluble and the solution is far from saturation.
Stirring or agitation can aid in the dissolution process, ensuring that the solute is evenly distributed throughout the solution. However, the solubility limit of the solute in the given solvent must be considered.
Exceeding the solubility limit will result in undissolved solute accumulating at the bottom of the container, without increasing the concentration of the solution itself.
Reverse Osmosis: A High-Pressure Solution
Reverse osmosis (RO) is a more sophisticated technique, often employed in water purification and industrial applications. This method utilizes pressure to force the solvent through a semi-permeable membrane, leaving the solute behind.
The membrane is designed to allow the passage of solvent molecules while blocking the larger solute molecules. This results in a concentrated solution on one side of the membrane and a purified solvent on the other.
RO is particularly useful for concentrating solutions containing solutes that are difficult to evaporate or heat-sensitive. However, it requires specialized equipment and careful control of pressure and flow rates.
Selective Precipitation: Isolating the Solute
Selective precipitation involves adding a chemical reagent that selectively reacts with the solute, forming an insoluble precipitate. This precipitate can then be separated from the solution, effectively removing the solute from the solvent.
After separation, the precipitate can be redissolved in a smaller volume of solvent to achieve a higher concentration. This technique is commonly used in purification processes and in the isolation of specific compounds.
Careful selection of the precipitating agent is crucial to ensure that it selectively targets the desired solute and does not introduce unwanted contaminants.
Factors Affecting Concentration Increase
The effectiveness of each concentration method depends on several factors, including the nature of the solute and solvent, temperature, pressure, and the presence of other substances. Understanding these factors is essential for optimizing the concentration process.
Temperature plays a crucial role in solubility; increasing the temperature often increases the solubility of solid solutes, allowing for a higher concentration to be achieved.
However, for gaseous solutes, increased temperature typically reduces solubility. Professor David Lee of MIT's Department of Chemical Engineering emphasizes that "a thorough understanding of the solute-solvent interactions is vital for selecting the appropriate concentration method."
Applications and Considerations
The ability to increase solution concentration is critical in various fields. In pharmaceuticals, precise concentrations are essential for drug efficacy and safety.
In environmental science, concentration techniques are used to analyze trace amounts of pollutants in water and soil samples. In manufacturing, concentrated solutions are often used as raw materials for various products.
When increasing concentration, it's crucial to consider the potential impact on the solution's properties, such as viscosity, density, and pH. These changes can affect the solution's behavior and its suitability for the intended application.
Future Trends in Concentration Techniques
Research is ongoing to develop more efficient and environmentally friendly concentration techniques. Nanotechnology is playing an increasingly important role, with the development of nanoscale membranes for selective separation and concentration.
Microfluidic devices offer the potential for highly controlled concentration processes, enabling precise manipulation of small volumes of solutions. These advancements are poised to revolutionize various industries, from pharmaceuticals to biotechnology.
The future of solution concentration lies in the development of innovative technologies that minimize energy consumption, reduce waste, and provide precise control over the concentration process. As Dr. Sharma notes, "the pursuit of more efficient and sustainable concentration methods is a critical area of research with broad societal implications."