Hplc Method Development Interview Questions & Answers

The pharmaceutical and analytical chemistry sectors are fiercely competitive, and landing a coveted role in HPLC method development demands more than just theoretical knowledge. Candidates must demonstrate practical experience, problem-solving skills, and a deep understanding of chromatographic principles. Preparing for these interviews requires a strategic approach, focusing on both technical proficiency and the ability to articulate complex concepts clearly.
This article delves into common HPLC method development interview questions, offering insightful answers and providing a framework for candidates to showcase their expertise. It provides essential guidance for aspiring analytical chemists, ensuring they are well-equipped to navigate the challenging interview process and secure their desired position. We will explore key areas, including method development strategies, troubleshooting techniques, and regulatory considerations.
Understanding the Fundamentals
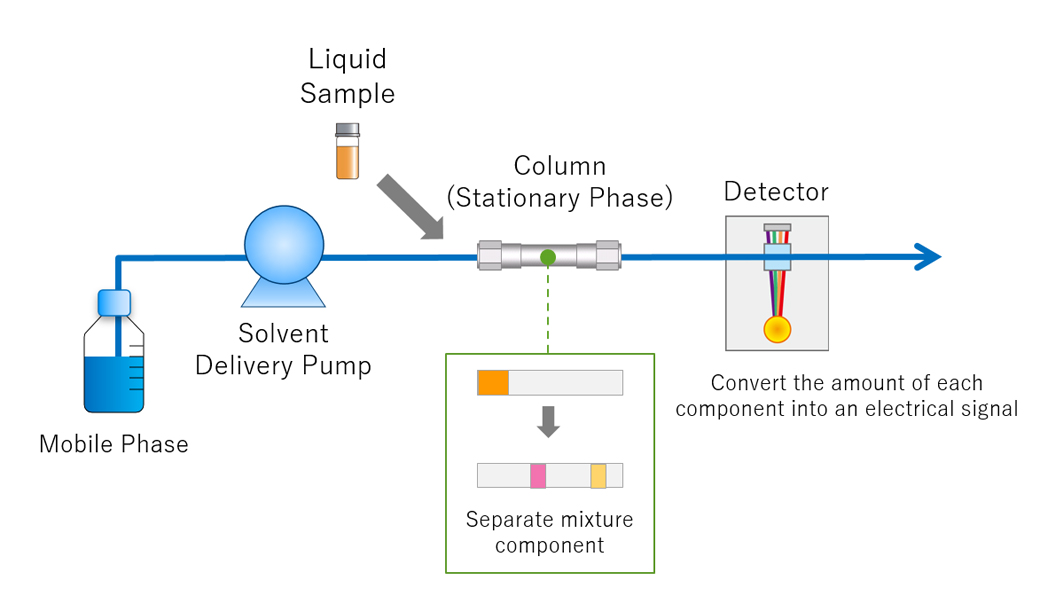
Question: Explain the key steps involved in HPLC method development.
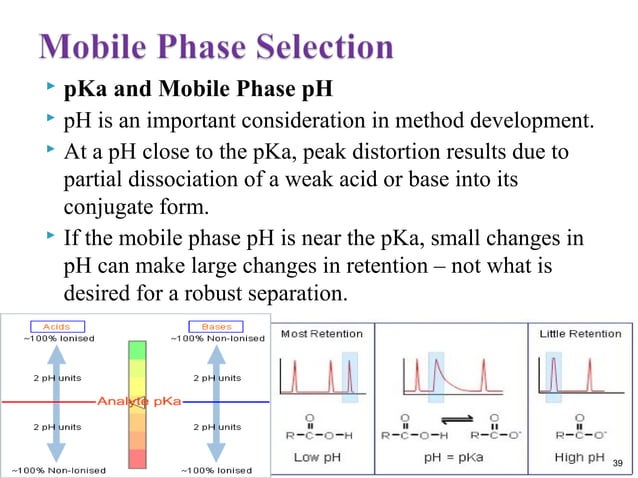
The first step is defining the analyte properties, including solubility, stability, and UV absorbance. Then, you select the appropriate stationary phase based on the analyte's characteristics, considering factors like polarity and molecular weight. Mobile phase optimization, involving solvent selection and gradient programming, is crucial for achieving adequate separation.
Question: What factors influence the choice of stationary phase in HPLC?
The choice of stationary phase depends largely on the analyte's polarity. For polar compounds, a polar stationary phase like silica or amino-bonded phases would be suitable. For non-polar compounds, a reversed-phase column like C18 or C8 is typically used.
Method Development Strategies
Question: Describe your approach to developing a robust HPLC method.
A robust method requires a systematic approach, starting with a Design of Experiments (DoE) to evaluate critical method parameters. This allows for identification of factors that significantly impact method performance, such as column temperature, flow rate, and mobile phase composition. Thorough method validation, following ICH guidelines, is essential to ensure accuracy, precision, and reproducibility.
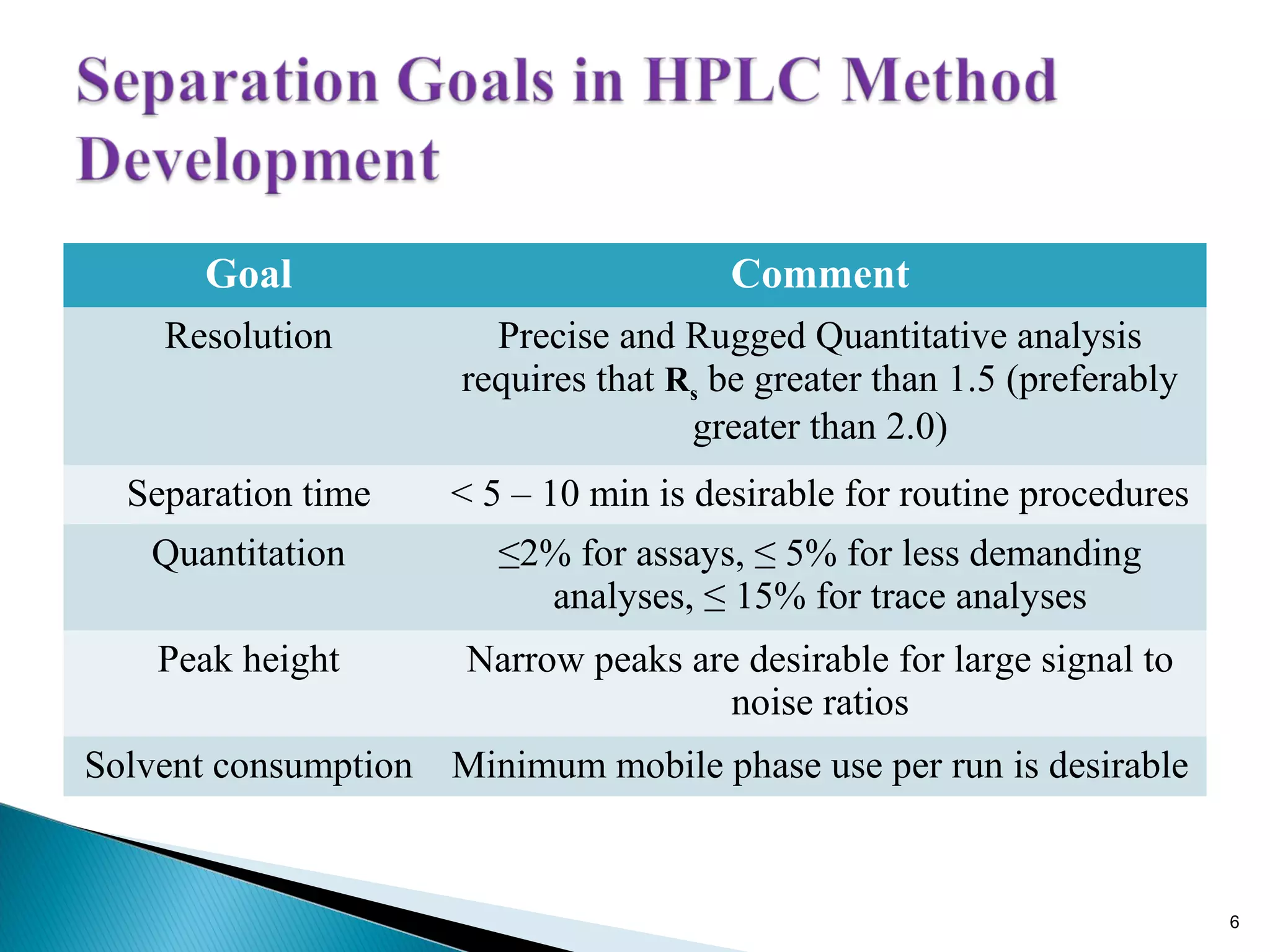
Question: How would you optimize a method for improved peak resolution?
Improving peak resolution can be achieved by adjusting several parameters. These parameters include optimizing the mobile phase composition to enhance selectivity, decreasing column temperature to improve retention, or using a column with a higher plate count. Another approach is to reduce the particle size of the stationary phase.
Troubleshooting and Problem Solving
Question: How would you troubleshoot a situation where you observe peak tailing?
Peak tailing can be caused by various factors, including silanol interactions on the stationary phase or column overload. To address this, consider adding a tailing suppressor to the mobile phase or lowering the sample concentration. Replacing the column with a higher-quality one can also be effective.
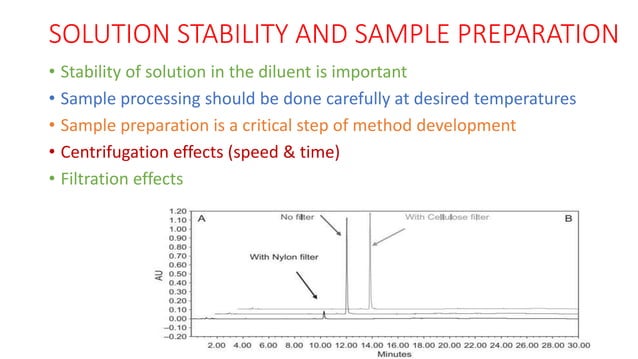
Question: What steps would you take if you encountered poor reproducibility in your HPLC method?
Poor reproducibility can stem from several sources, such as inconsistent sample preparation or variations in instrument performance. First, verify the accuracy of the instrument parameters, including flow rate and injection volume. Then, ensure proper sample preparation techniques and calibrate the HPLC system to minimize variability.
Regulatory Considerations and Compliance
Question: Explain the importance of method validation in the pharmaceutical industry.
Method validation is critical for ensuring the reliability and accuracy of analytical methods used in pharmaceutical manufacturing and quality control. It provides documented evidence that the method is suitable for its intended purpose and meets regulatory requirements, such as those set forth by the FDA and ICH. Method validation ensures the quality and safety of pharmaceutical products.
Question: What are the key parameters to be evaluated during method validation?
Key parameters to be evaluated during method validation include selectivity, linearity, accuracy, precision, limit of detection (LOD), limit of quantitation (LOQ), and robustness. These parameters assess the method's ability to accurately and reliably measure the analyte of interest within a specific matrix. These parameters confirm the method's suitability for its intended application.
Beyond the Technical
Demonstrating a strong understanding of HPLC principles is crucial, but successful candidates also possess excellent communication and problem-solving abilities. Be prepared to discuss specific examples from your experience where you successfully developed or optimized an HPLC method, highlighting the challenges you faced and the solutions you implemented. Emphasize your commitment to continuous learning and staying abreast of the latest advancements in chromatographic techniques.
Mastering these key concepts and practicing clear articulation of your knowledge will significantly enhance your interview performance. Remember to showcase your enthusiasm for analytical chemistry and your willingness to contribute to the advancement of chromatographic science. Success in HPLC method development interviews hinges on a blend of technical expertise, problem-solving skills, and effective communication.