Is Vinegar A Compound Or Mixture

Imagine strolling through a sun-drenched orchard, the air thick with the scent of ripe apples. Now, picture those apples transforming, almost magically, into a tangy liquid that adds a zesty kick to your salad or cleans your countertops with surprising efficiency. This liquid, of course, is vinegar – a household staple found in kitchens and cleaning cabinets around the world. But have you ever stopped to ponder its fundamental nature? Is it a simple substance, a compound meticulously formed from combined elements, or a more complex mixture of different ingredients mingling together?
The question of whether vinegar is a compound or a mixture is a surprisingly nuanced one. This article will delve into the chemical composition of vinegar, exploring its origins, its essential ingredients, and the scientific principles that define its classification.
The Essence of Vinegar: Beyond the Tang
Vinegar's story begins with fermentation, a process as old as civilization itself. It is the magic of microorganisms transforming sugars into acids.
Typically, this involves a two-step process. First, yeast converts sugars (like those found in fruit juice or grains) into alcohol, primarily ethanol.
Then, a group of bacteria called Acetobacter takes over, oxidizing the alcohol into acetic acid – the key ingredient that gives vinegar its characteristic sour taste and pungent aroma.
Unveiling the Composition
At its heart, vinegar is an aqueous solution, meaning it's primarily water (H₂O). Dissolved within this water are other components, most notably acetic acid (CH₃COOH).
The concentration of acetic acid typically ranges from 4% to 8% in table vinegar, though it can be much higher in pickling vinegars. Other substances present in smaller quantities contribute to vinegar’s flavor and color.
These can include trace amounts of mineral salts, esters, and other organic acids like tartaric acid (especially in wine vinegars).
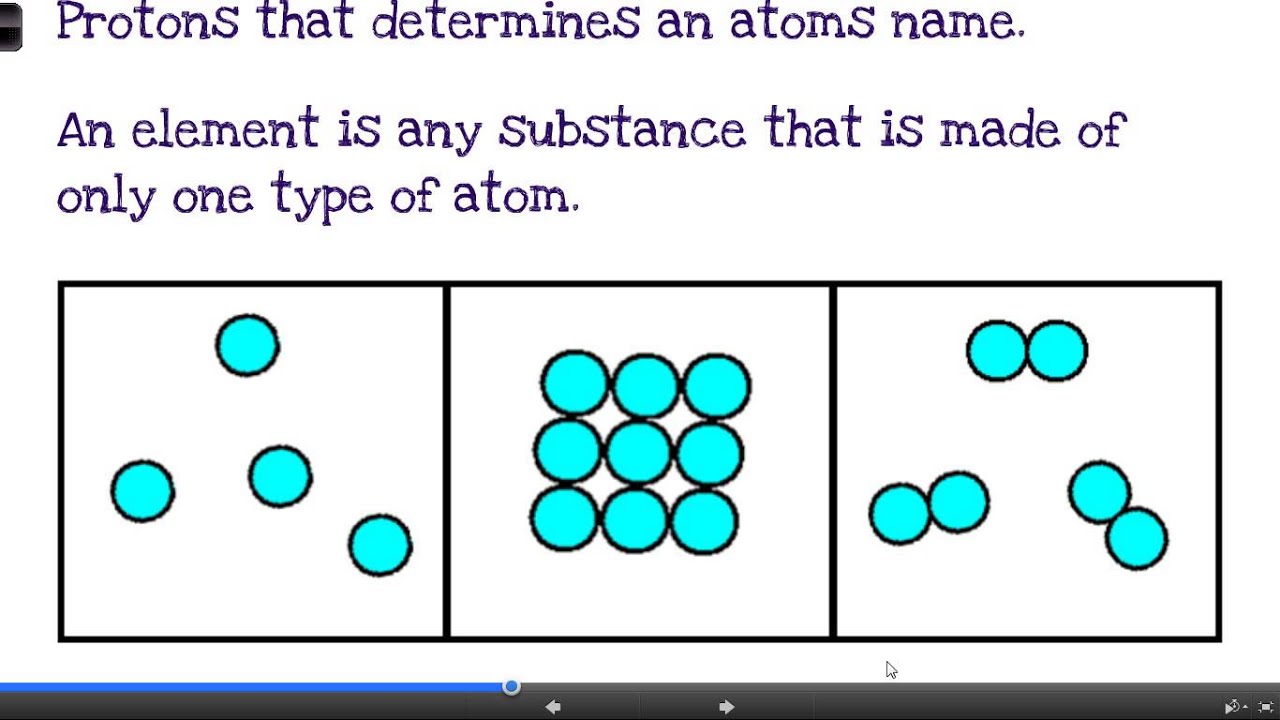
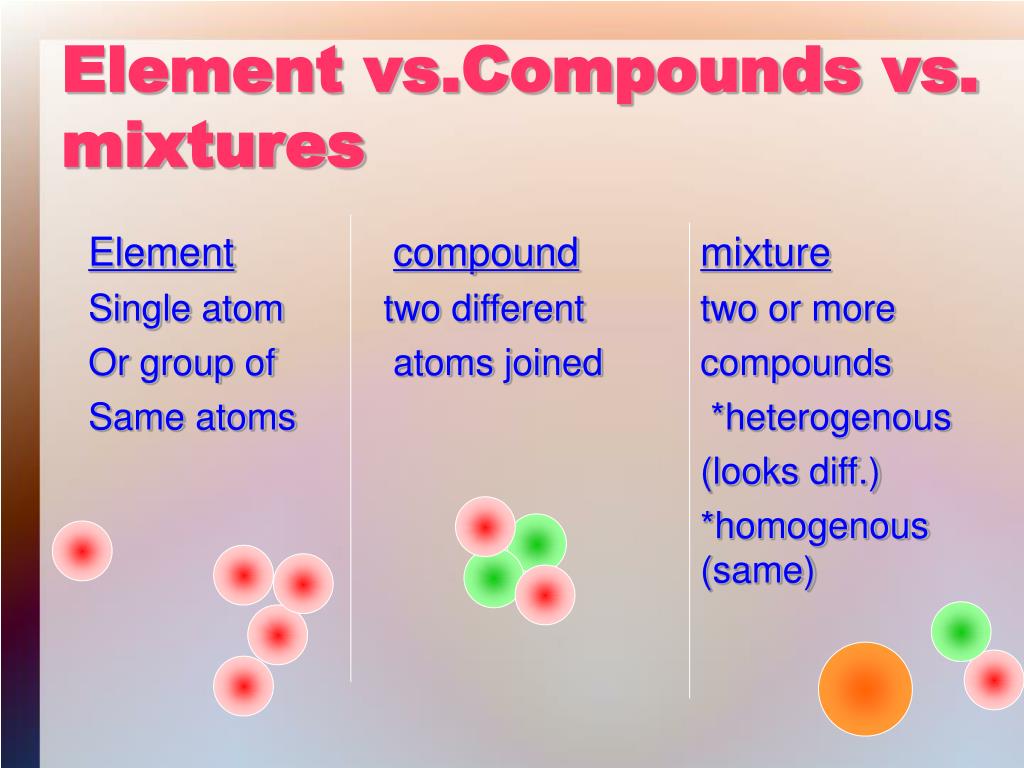
Compound vs. Mixture: Defining the Terms
To understand vinegar’s classification, we need to define the terms. A compound is a substance formed when two or more elements are chemically bonded together in a fixed ratio. Water (H₂O) and carbon dioxide (CO₂) are prime examples.
A mixture, on the other hand, is a combination of two or more substances that are physically combined but not chemically bonded. Air, which is a mixture of nitrogen, oxygen, and other gases, is a classic example.
In a mixture, the individual components retain their own chemical properties and can, in principle, be separated by physical means.
Why Vinegar is a Mixture
Given these definitions, vinegar clearly falls into the category of a mixture. Acetic acid and water are not chemically bonded to each other.
They are simply mixed together, and the ratio of acetic acid to water can vary depending on the type of vinegar. The fact that vinegar is a mixture is further supported by the presence of other, variable components beyond acetic acid and water.
These additional substances are not chemically bound to either water or acetic acid and their concentration varies.
The Many Faces of Vinegar: A World of Variety
The world of vinegar is far more diverse than most people realize. While white distilled vinegar, made from grain alcohol, is the most common type, many other varieties exist, each with its unique flavor profile and production method.
Apple cider vinegar, made from fermented apple juice, boasts a fruity sweetness. Balsamic vinegar, aged in wooden barrels, offers a complex, syrupy taste.
Wine vinegars, both red and white, impart the characteristic notes of their respective wines.
Beyond the Kitchen: Vinegar's Versatile Applications
Vinegar's uses extend far beyond the culinary realm. Its acidic properties make it an effective cleaning agent, capable of dissolving mineral deposits and killing bacteria.
Gardeners often use it to control weeds and adjust soil pH. In traditional medicine, it has been used for various ailments.
Its versatility underscores its importance as a readily available and environmentally friendly resource.
The Scientific Significance
Understanding whether vinegar is a compound or a mixture is more than just a matter of scientific curiosity; it’s a lesson in fundamental chemistry. It reinforces the distinction between substances formed through chemical bonding and those that are simply physically combined.
This concept is critical for understanding the properties of matter and how different substances interact with each other.
From pharmaceuticals to materials science, this understanding underlies a wide range of scientific disciplines.
Moreover, the production of vinegar serves as a practical example of fermentation, a biotechnological process with wide-ranging applications in the food, beverage, and pharmaceutical industries. As Dr. Emily Carter, a professor of Chemistry at UCLA, stated in a 2020 interview with Scientific American, "Vinegar is a fantastic example of how natural processes can be harnessed to create useful products. The simple act of fermentation, transforming sugars into acids, represents a powerful chemical transformation with immense practical applications."
Conclusion: Appreciating the Everyday
So, the next time you reach for that bottle of vinegar, take a moment to appreciate its composition. It’s not a simple, singular substance, but a carefully balanced mixture, a testament to the power of natural processes and human ingenuity.
It's a reminder that even the most ordinary objects can hold fascinating scientific secrets, waiting to be uncovered.
From its humble origins to its versatile applications, vinegar exemplifies the beauty and complexity that can be found in the everyday world around us, and its composition as a mixture underpins many of its useful properties.


![Is Vinegar A Compound Or Mixture Is Vinegar An Element, Compound, or Mixture? [ANSWERED] – Dear Learners](https://dearlearners.com/wp-content/uploads/2021/01/is-vinegar-an-element-compound-or-mixture_.jpg)