Isotopes Of An Atom Differ In

Invisible yet fundamental, isotopes are the subtle variations within the building blocks of all matter. Their differences, residing not in the type of element they constitute, but in the very core of their atoms, hold the key to understanding everything from the age of the Earth to the intricacies of medical diagnostics.
This seemingly minor atomic distinction – differing numbers of neutrons – dictates not only the mass of an atom, but also profoundly impacts its stability and radioactive properties. Understanding the nuances of isotopes is critical for advancements in various fields, but also presents challenges in areas like nuclear waste management and the ethical use of radioactive materials.
What are Isotopes?
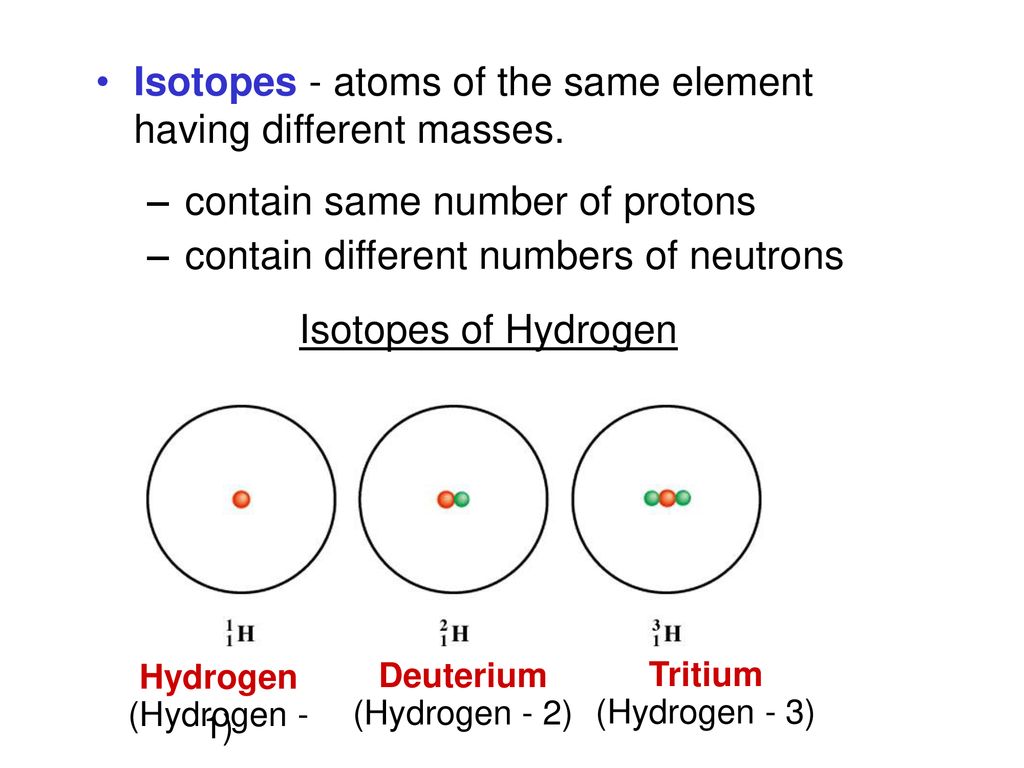
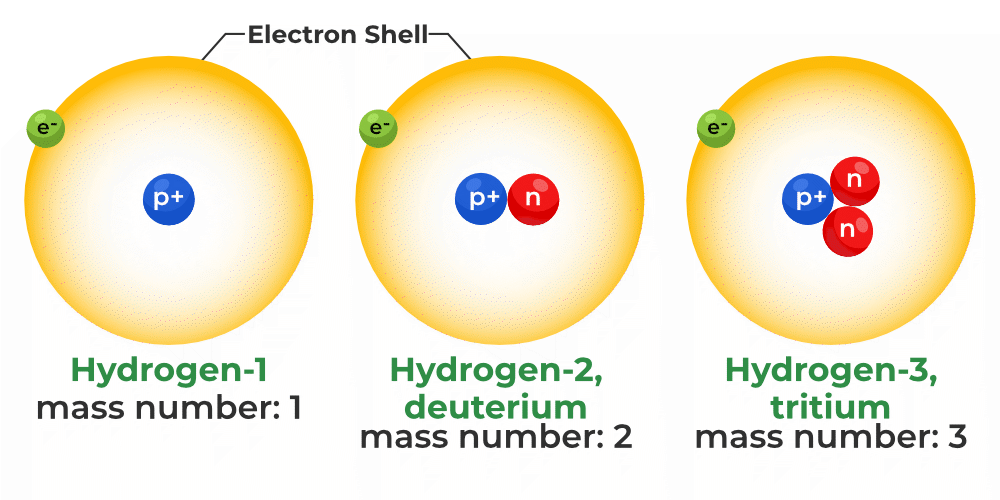
At the heart of every atom lies the nucleus, a dense region comprised of protons and neutrons. Protons define the element: an atom with one proton is hydrogen, while one with six protons is carbon.
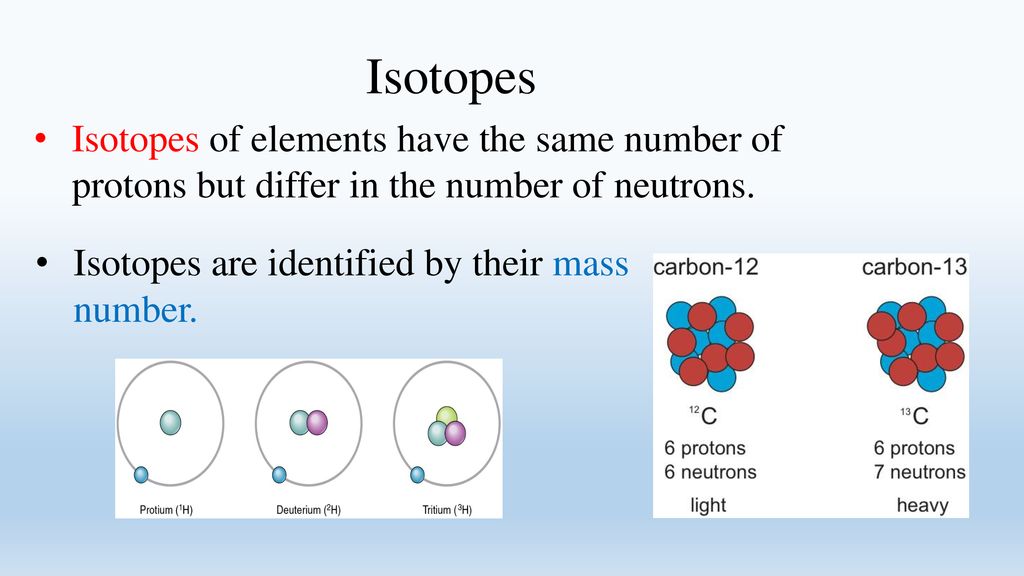
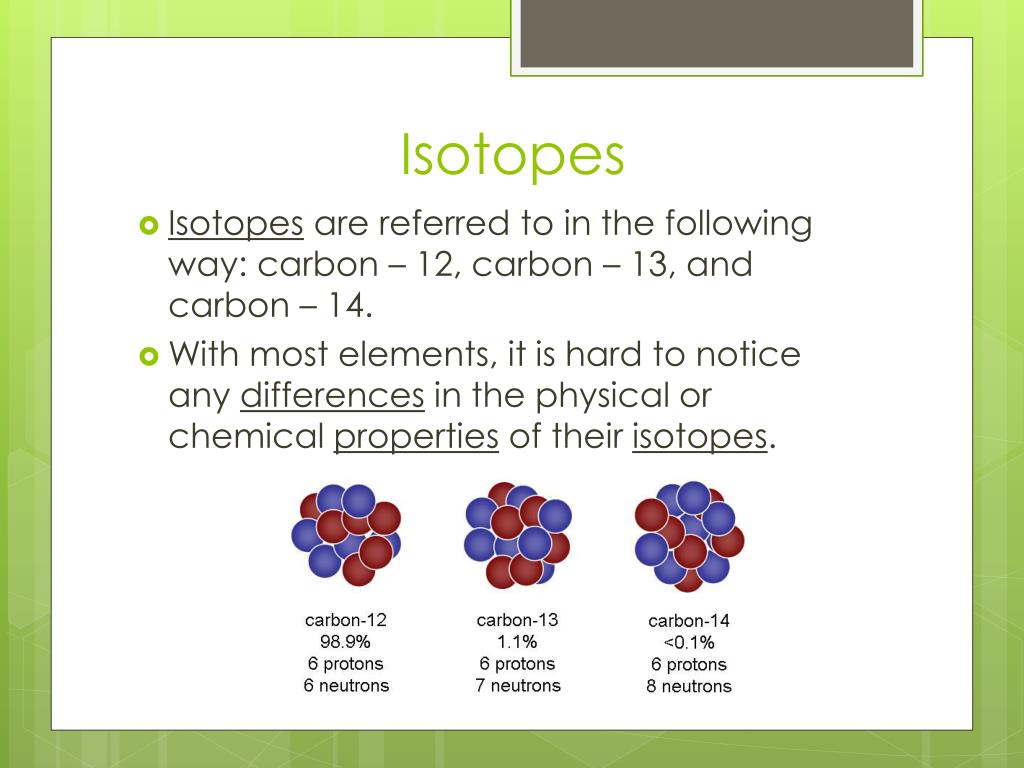
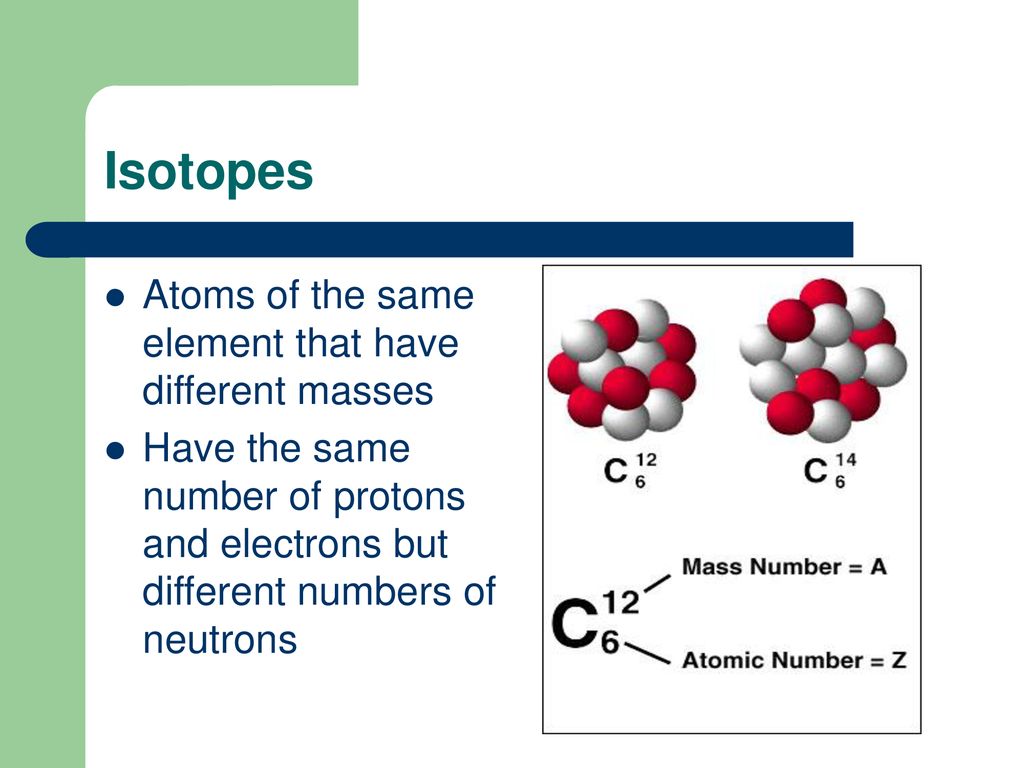
Isotopes are variants of the same element that have the same number of protons but different numbers of neutrons. For example, carbon-12 (12C) has six protons and six neutrons, while carbon-14 (14C) has six protons and eight neutrons.
Because they have the same number of protons, isotopes of an element exhibit nearly identical chemical behavior. However, their differing neutron counts lead to significant variations in their atomic mass and stability, affecting their radioactive properties.
The Significance of Neutron Number
The number of neutrons within an atom's nucleus plays a crucial role in determining its stability. Certain ratios of protons to neutrons are more stable than others.
Isotopes with an unstable nucleus undergo radioactive decay, transforming into a different element or isotope while releasing energy in the form of radiation. This decay process is characterized by a specific half-life, which is the time it takes for half of the radioactive atoms in a sample to decay.
Radioactive isotopes, also known as radioisotopes, are widely used in medicine, industry, and research due to their predictable decay patterns and the energy they release.
Applications Across Diverse Fields
Dating the Past
Carbon-14 dating, a technique relying on the decay of the carbon-14 isotope, is an indispensable tool for archaeologists and paleontologists. By measuring the remaining amount of carbon-14 in organic materials, scientists can determine the age of fossils and artifacts up to approximately 50,000 years old.
Other radioactive isotopes, such as uranium-238 and potassium-40, with their much longer half-lives, are used to date rocks and geological formations, providing insights into the Earth's history over millions of years.
Medical Diagnostics and Treatment
Radioisotopes play a critical role in medical imaging and therapy. Technetium-99m, for example, is a widely used radioisotope in diagnostic imaging, allowing doctors to visualize internal organs and detect abnormalities.
In cancer treatment, radioactive isotopes like iodine-131 are used to target and destroy cancerous cells. Targeted Alpha Therapy (TAT) is an emerging approach that utilizes alpha-emitting isotopes to selectively kill cancer cells with minimal damage to surrounding healthy tissues.
Industrial Applications
In industry, isotopes are used for a variety of purposes, including gauging the thickness of materials, tracing the flow of liquids and gases, and sterilizing medical equipment. Radioactive tracers are also used to detect leaks in pipelines and to monitor the efficiency of industrial processes.
Challenges and Ethical Considerations
While isotopes offer numerous benefits, their use also presents challenges. Radioactive materials can be hazardous to human health and the environment, requiring careful handling and disposal.
The management of nuclear waste, which contains long-lived radioactive isotopes, is a significant environmental challenge. Finding safe and permanent disposal solutions for nuclear waste remains a pressing concern worldwide.
The potential for misuse of radioactive materials, such as in radiological weapons, also raises security concerns. International regulations and monitoring programs are in place to prevent the diversion of radioactive materials for malicious purposes.
The Future of Isotope Research
Ongoing research is focused on developing new and improved methods for producing and utilizing isotopes. Scientists are exploring novel techniques for separating isotopes, creating new radioisotopes for medical and industrial applications, and developing more effective strategies for nuclear waste management.
The Facility for Rare Isotope Beams (FRIB) at Michigan State University, for example, is a state-of-the-art research facility dedicated to the study of rare isotopes. FRIB allows scientists to probe the structure of atomic nuclei, understand the origin of the elements, and explore new applications of isotopes.
“Isotope research holds immense potential to transform various fields, from medicine and energy to materials science and national security,” says Dr. Emily Carter, a leading researcher in isotope technology. “By continuing to unravel the mysteries of the atomic nucleus, we can unlock new possibilities for addressing some of the world's most pressing challenges.”
Conclusion
The seemingly simple difference in the number of neutrons within an atom defines isotopes, and this difference underpins a vast array of scientific advancements. From dating ancient artifacts to treating life-threatening diseases, isotopes are essential tools in a diverse range of fields. As research progresses, we can expect even more innovative applications of isotopes to emerge, offering solutions to some of humanity's greatest challenges, even as we grapple with the responsibility of handling them safely and ethically.











.jpg)