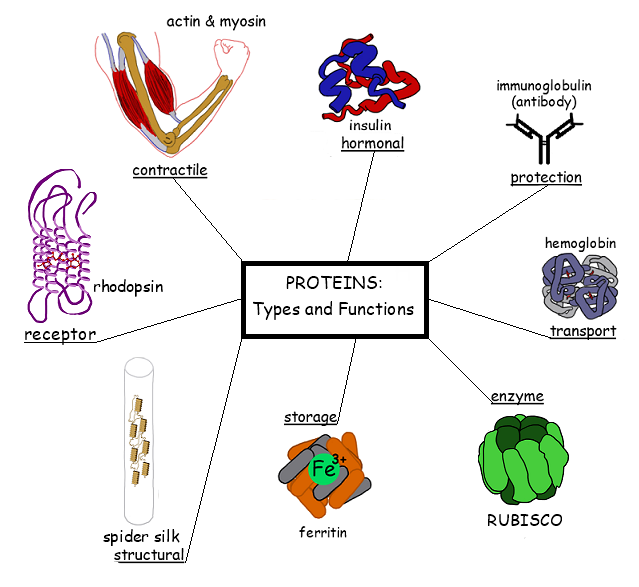
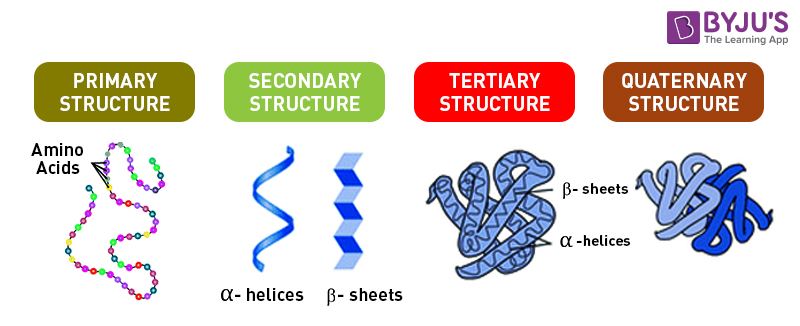
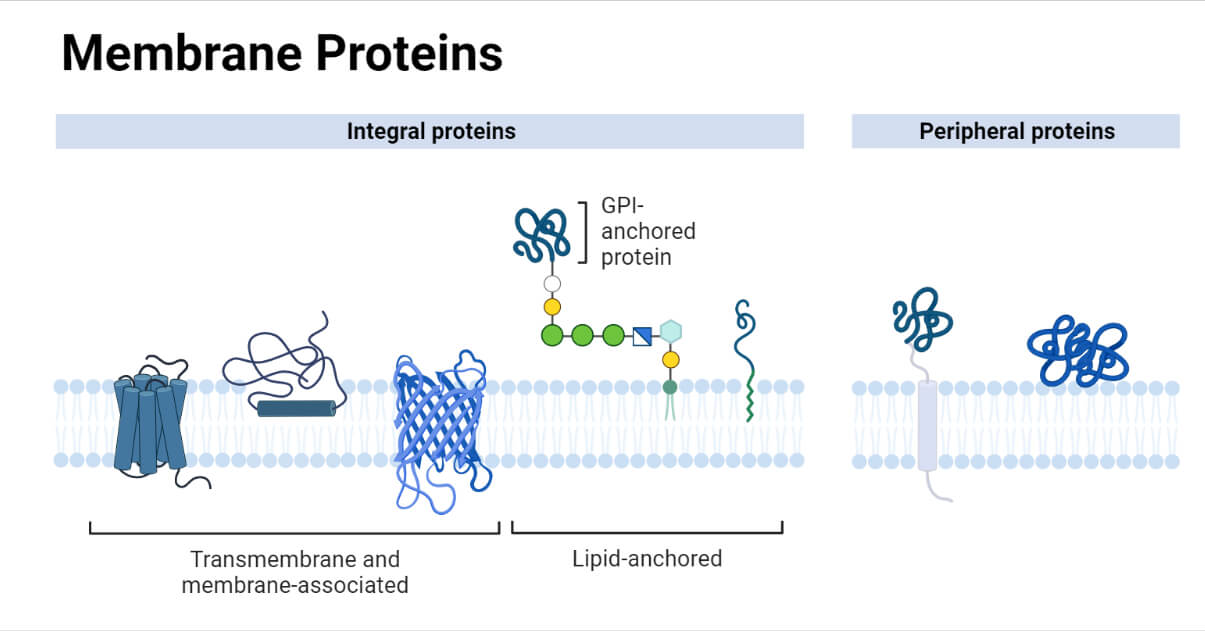
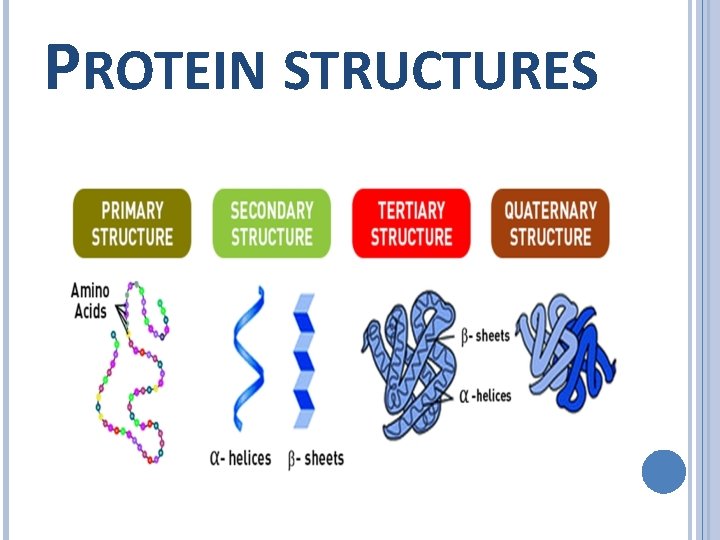
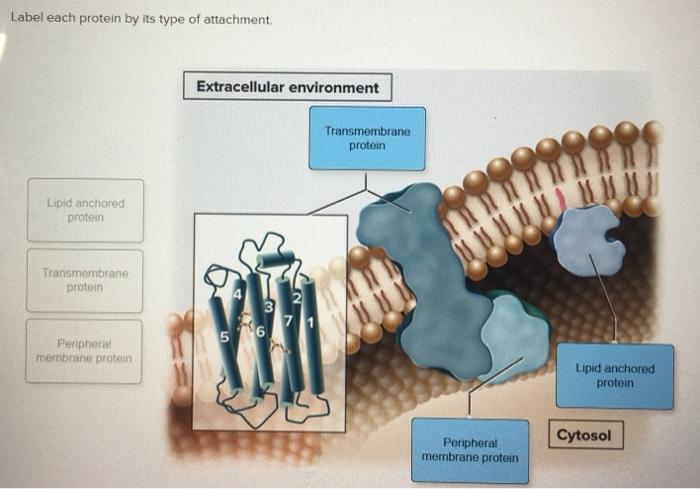
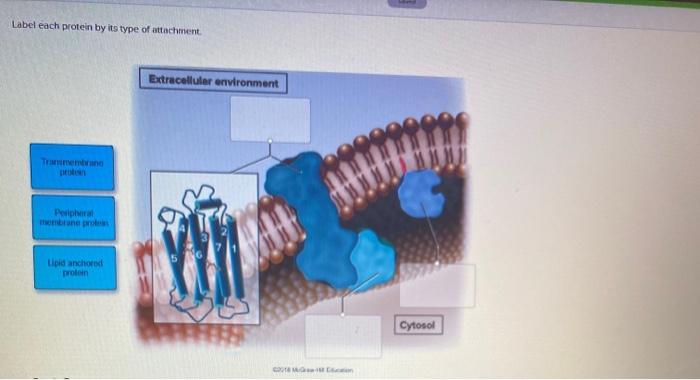
Label Each Protein By Its Type Of Attachment
Scientists have achieved a significant breakthrough in proteomics by developing a new method for precisely labeling proteins according to their type of attachment, a development poised to revolutionize drug discovery, disease diagnostics, and our fundamental understanding of cellular processes.
This innovative technique, described in a recent publication in Nature Methods, allows researchers to distinguish between different types of protein modifications with unprecedented accuracy and speed. The method has the potential to accelerate research in various biomedical fields.
The Significance of Protein Attachments
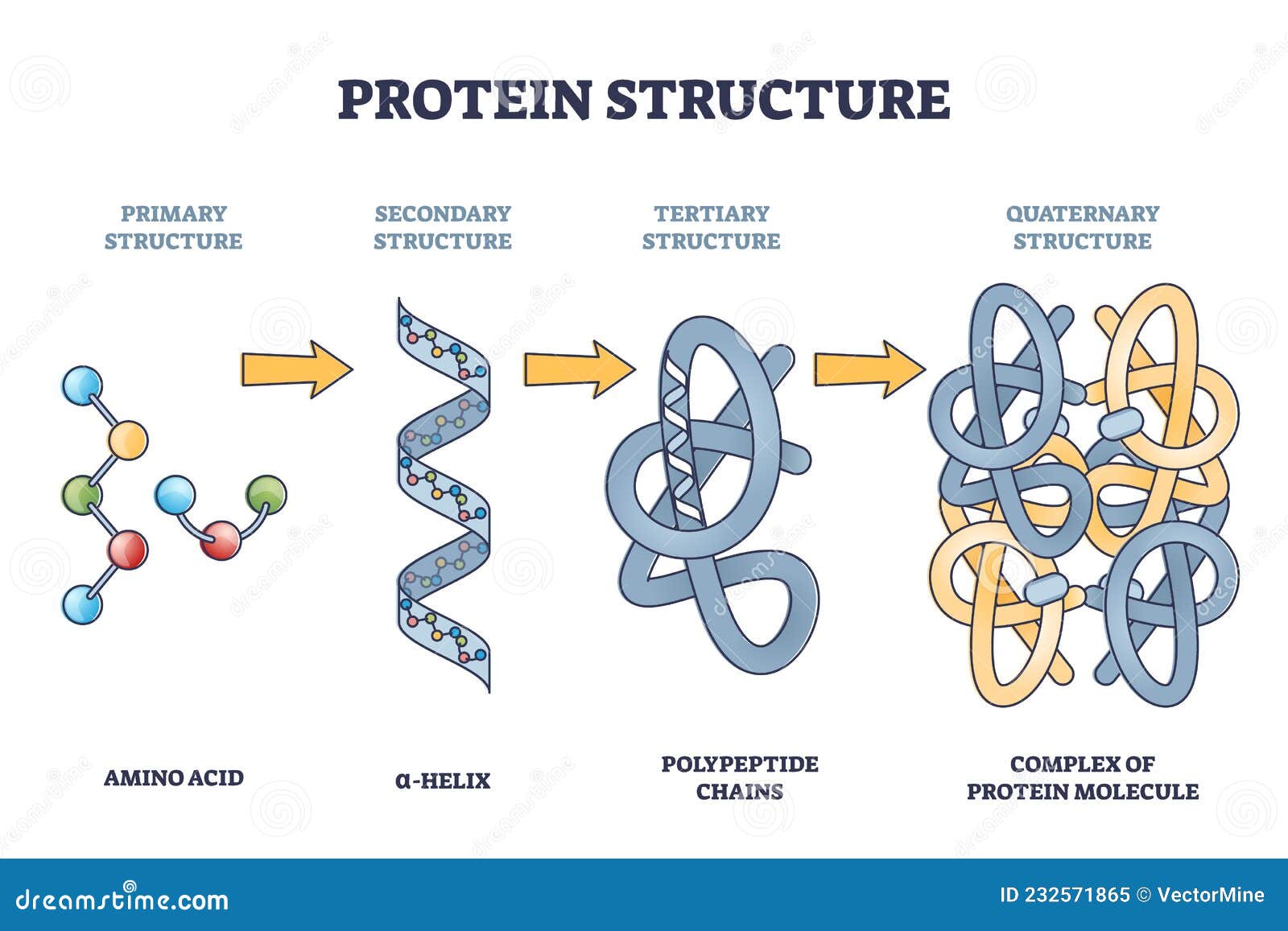
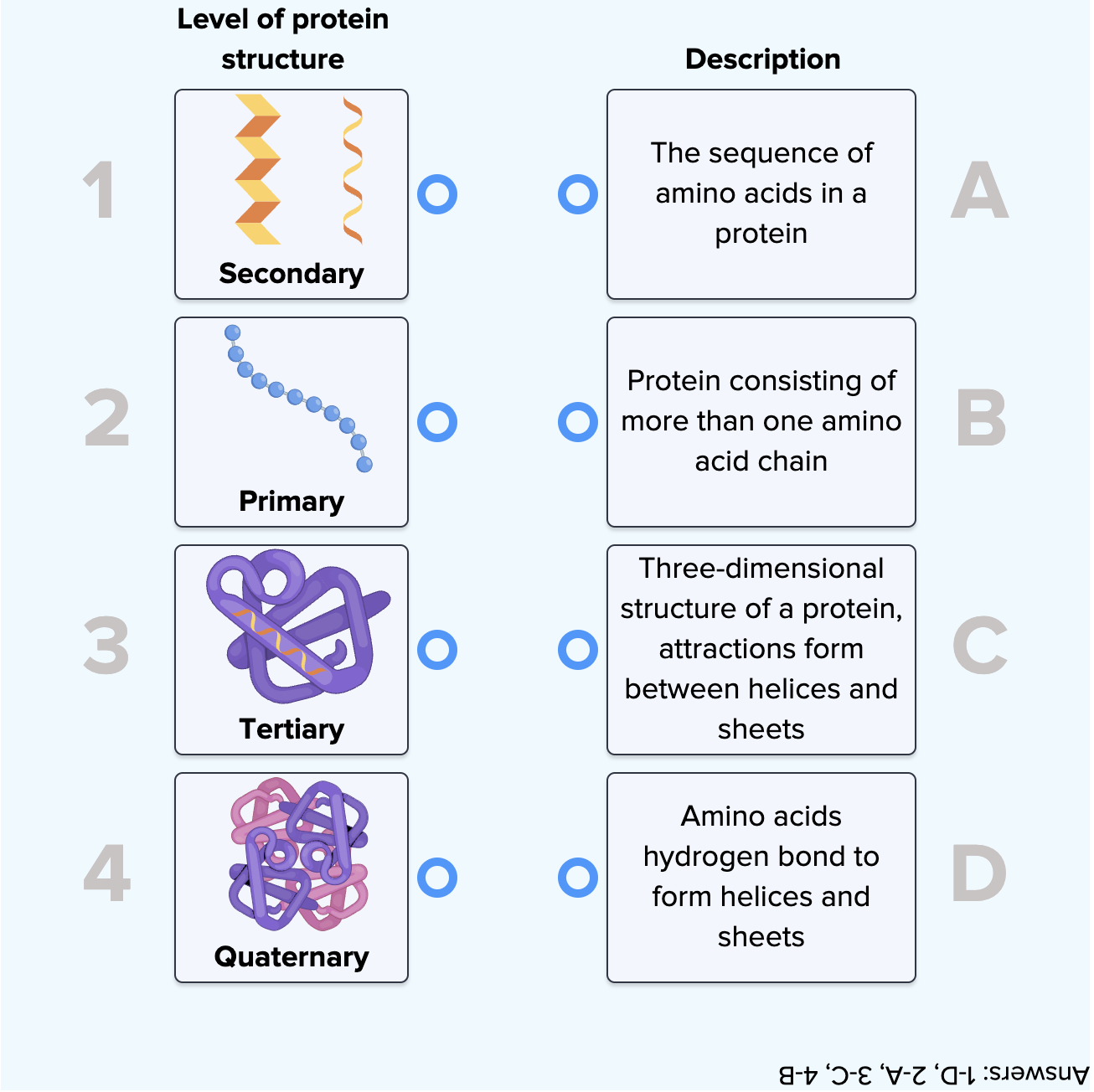
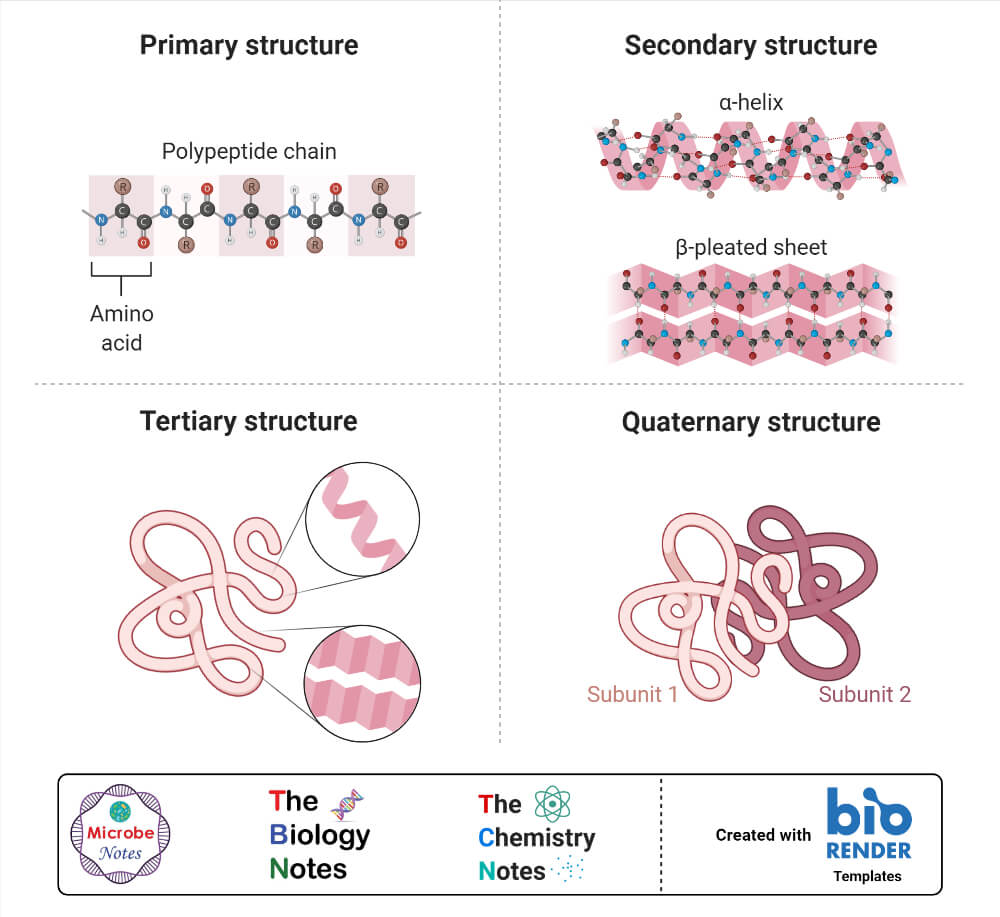
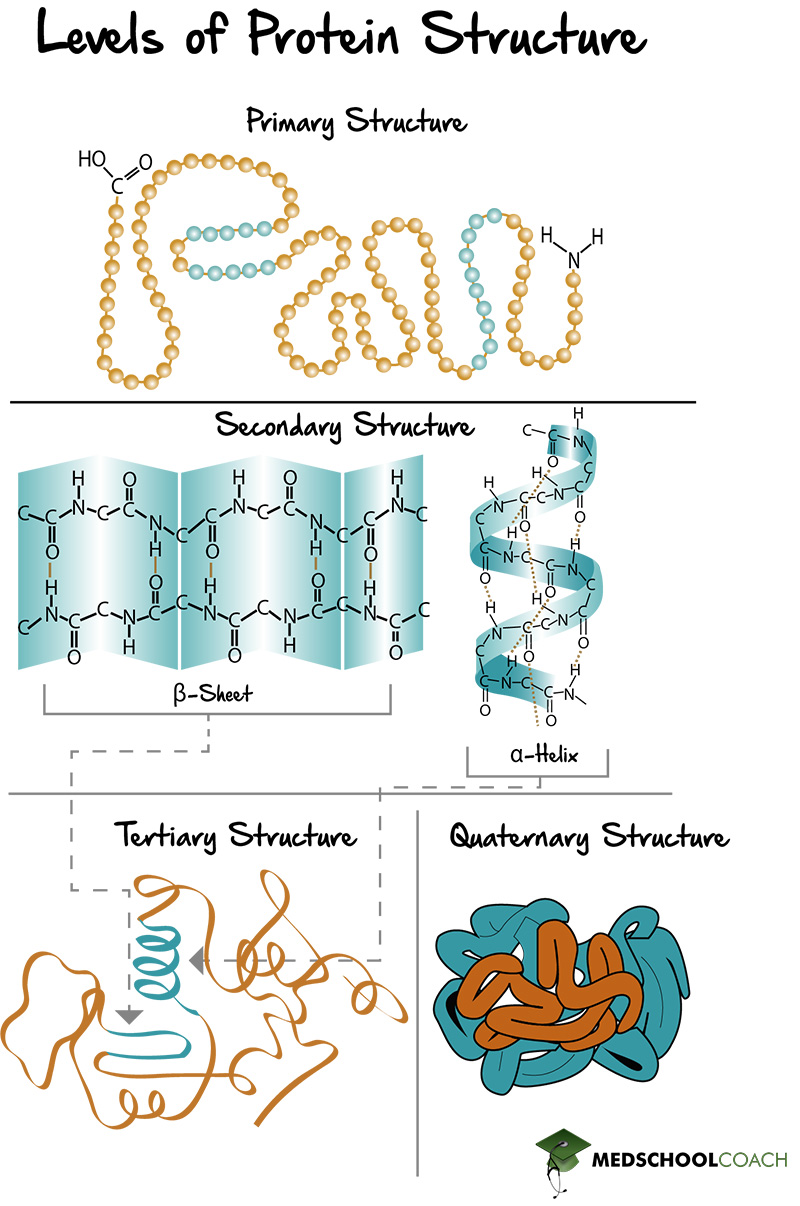
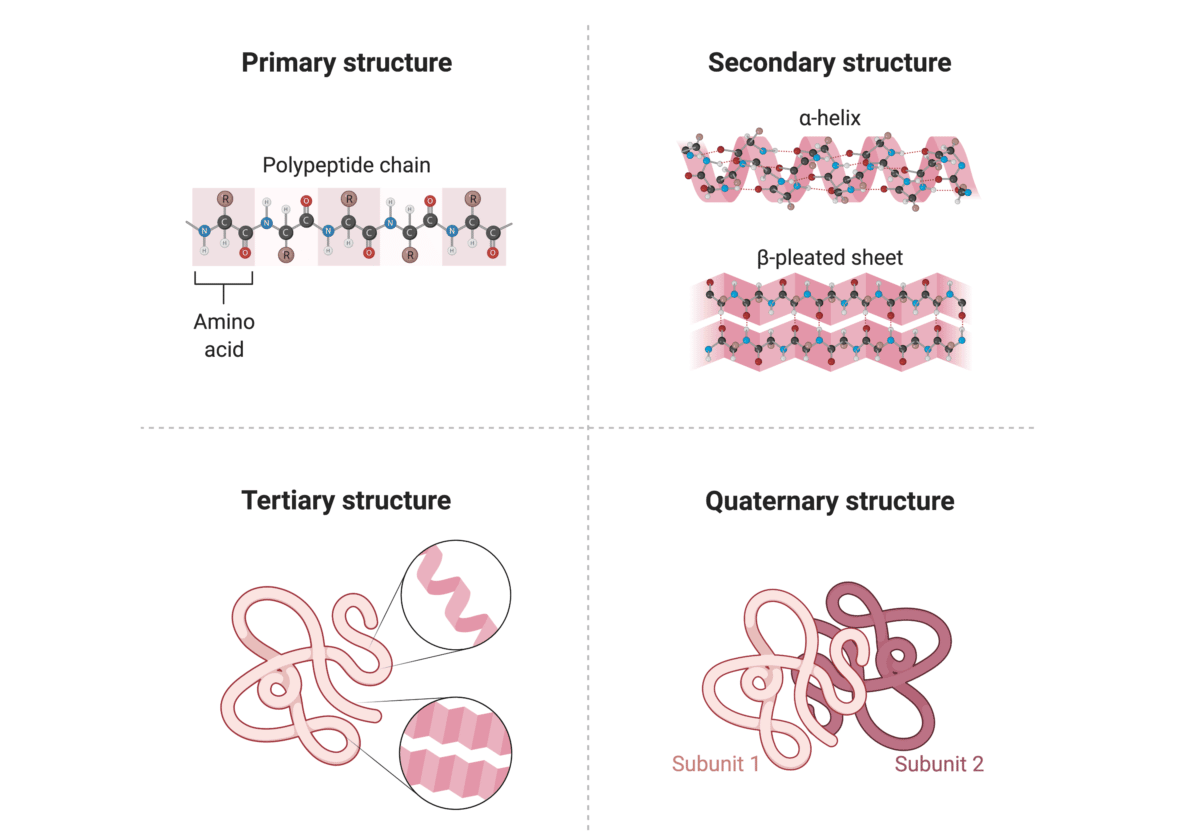
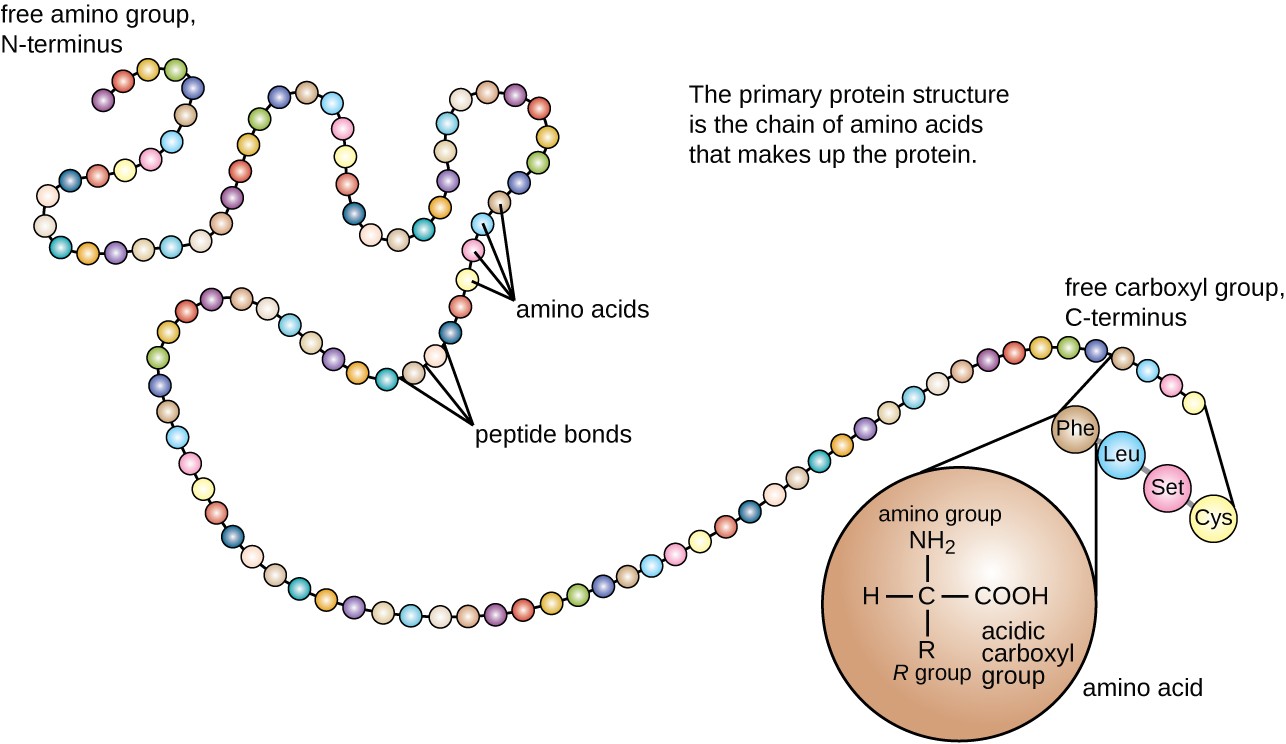
Proteins are the workhorses of our cells, carrying out a vast array of functions essential for life. These functions are often regulated by the attachment of other molecules, such as sugars (glycosylation), lipids (lipidation), or phosphate groups (phosphorylation).
These attachments, known as post-translational modifications (PTMs), can dramatically alter a protein's activity, location, and interactions with other molecules. Aberrant PTMs are implicated in a wide range of diseases, including cancer, Alzheimer's disease, and diabetes.
Therefore, accurately identifying and characterizing these attachments is crucial for understanding disease mechanisms and developing targeted therapies. Understanding how protein attachments affect protein function at a granular level is a critical scientific endeavor.
The New Labeling Method: A Game Changer
The newly developed labeling method, spearheaded by a team at the Broad Institute of MIT and Harvard, employs a combination of chemical labeling and mass spectrometry to identify proteins and their attachments. The team was led by Dr. Alice Chen, a leading expert in proteomics.
The key innovation lies in the use of specially designed chemical tags that selectively bind to specific types of protein attachments. These tags are then used to isolate and identify the modified proteins using mass spectrometry.
Unlike existing methods, which often struggle to distinguish between closely related modifications, the new technique offers high specificity and sensitivity. This leads to a much more comprehensive and accurate picture of the protein modification landscape.
Key Details of the Methodology
The process begins with preparing a cell lysate, which contains a mixture of all the proteins in the cell. The researchers then introduce the chemical tags, each designed to react with a specific type of protein attachment.
After the tags have bound to their target modifications, the proteins are digested into smaller peptides. These peptides are then analyzed by mass spectrometry, which can identify the peptides and determine the location and type of attachment.
The Dr. Chen's team has also developed sophisticated software to analyze the mass spectrometry data and generate a detailed map of protein modifications. This automated analysis greatly speeds up the process and reduces the risk of human error.
Impact and Applications
The potential impact of this new labeling method is immense. One of the most promising applications is in drug discovery. This method could provide a new way to identify and validate drug targets.
By identifying the specific protein modifications that are associated with a disease, researchers can develop drugs that specifically target those modifications. The method can allow scientist to test drug efficacies.
This could lead to more effective and less toxic therapies. For example, in cancer research, the method could be used to identify proteins that are abnormally phosphorylated in cancer cells, allowing researchers to develop kinase inhibitors that specifically target these proteins.
Diagnostic Potential
The new method also has significant potential for disease diagnostics. By analyzing the protein modification profiles of patient samples, doctors could potentially diagnose diseases at an earlier stage and with greater accuracy.
For example, the method could be used to detect early signs of Alzheimer's disease by identifying changes in the phosphorylation patterns of specific proteins in cerebrospinal fluid. Furthermore, the method can be used to monitor disease progression.
This could lead to earlier interventions and improved patient outcomes. Early detection and treatment will benefit the patient.
Fundamental Research
Beyond drug discovery and diagnostics, the new labeling method will also be a valuable tool for fundamental research. Researchers can now study the roles of protein modifications in a wide range of cellular processes, such as cell signaling, DNA repair, and protein trafficking.
This could lead to a deeper understanding of the basic mechanisms of life. This will allow scientist to discover new biological processes.
“This new technology will revolutionize our understanding of the complexity of protein modifications. It provides a powerful tool to dissect the molecular mechanisms underlying various biological processes and diseases," says Dr. Maria Rodriguez, a proteomics expert at the National Institutes of Health.
The Human Angle
For Dr. Chen, the development of this new method is the culmination of years of dedicated research. She recalls the challenges faced by her team in developing the chemical tags and optimizing the mass spectrometry analysis.
"There were many times when we thought we had hit a dead end," she says. "But we persevered, driven by the belief that we could make a real difference in the fight against disease."
The team's hard work has paid off, and their new method is poised to make a significant impact on the lives of patients around the world.
Conclusion
The development of a new method for precisely labeling proteins according to their type of attachment marks a major advance in proteomics. The potential of this method to revolutionize drug discovery, disease diagnostics, and our fundamental understanding of cellular processes is immense. As researchers continue to explore its applications, we can expect to see significant progress in the fight against a wide range of diseases.
/protein-structure-373563_final11-5c81967f46e0fb00012c667d.png)