Latent Heat Of Ice To Water
Imagine a crisp winter morning. The world is draped in a blanket of snow, glistening under the pale sunlight. You step outside, and the air bites at your cheeks, a stark reminder of the powerful forces at play beneath the seemingly static landscape. But what if I told you that even as the sun warms the ice, an invisible energy is working, silently but profoundly, to transform the world around you?
This invisible energy is known as latent heat, and it's the key to understanding how ice transforms into water. While this might seem like a simple high school science lesson, the principles of latent heat have far-reaching implications, from understanding global climate patterns to designing efficient cooling systems.
The Magic of Phase Change
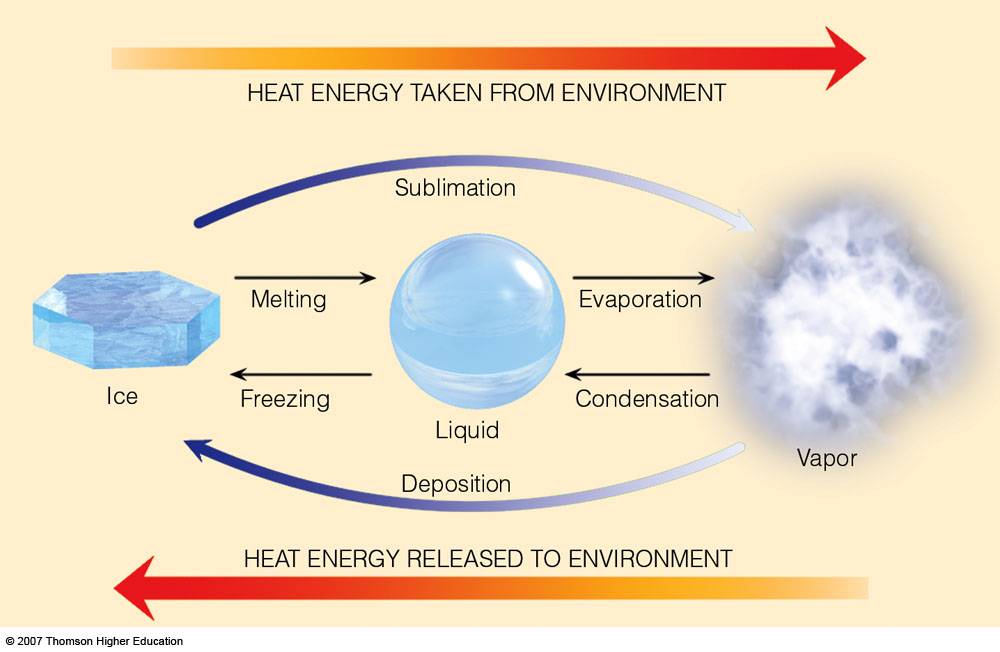
Let's delve into the fascinating world of phase transitions. Matter, as we know it, exists in different states: solid, liquid, and gas.
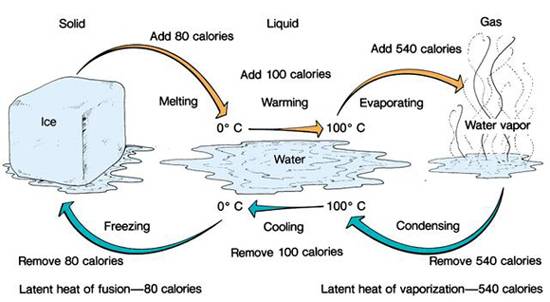
The transition between these states, such as ice melting into water, involves a transfer of energy. This is where latent heat comes into play.
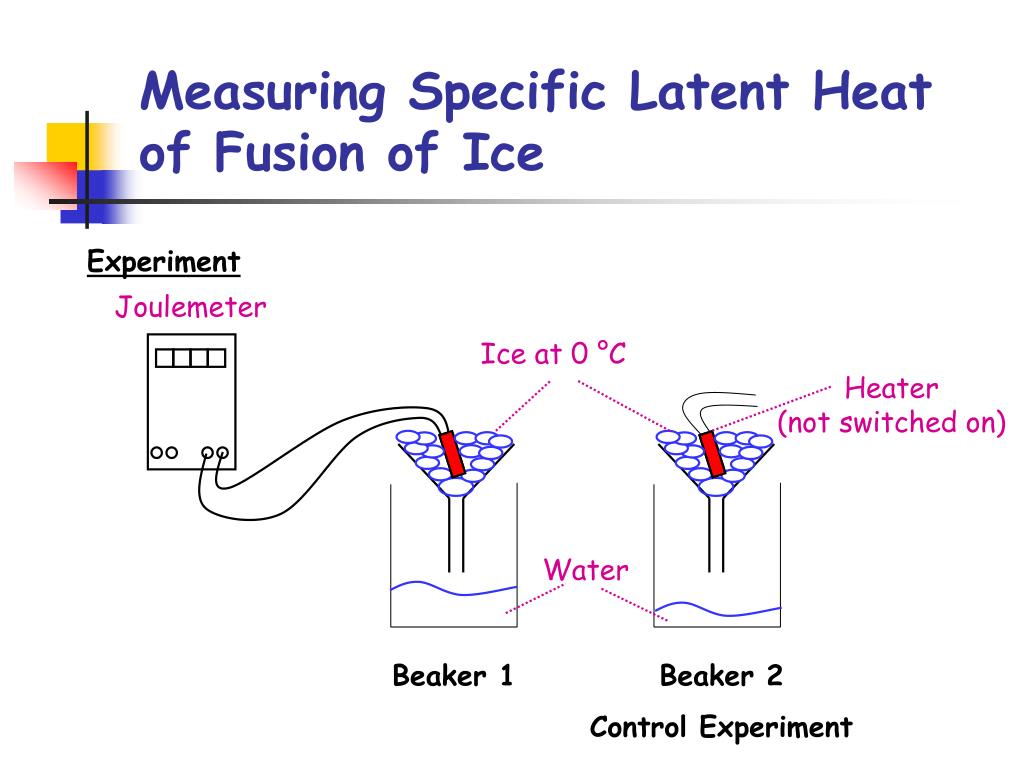
Specifically, the latent heat of fusion is the energy required to change a substance from a solid to a liquid at a constant temperature.
Breaking the Bonds
Think of ice as a tightly knit community of water molecules. They are bound together by strong hydrogen bonds.
When you heat ice, you're essentially giving these molecules energy to break free from their bonds.
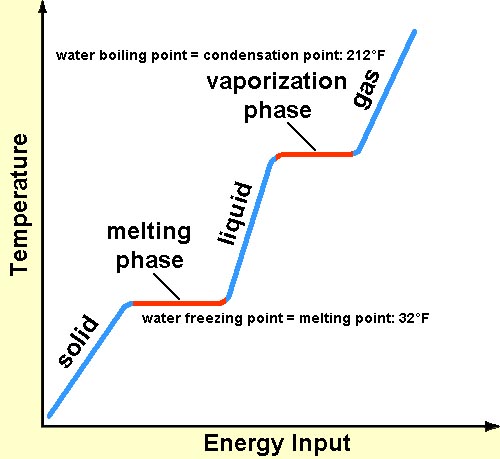
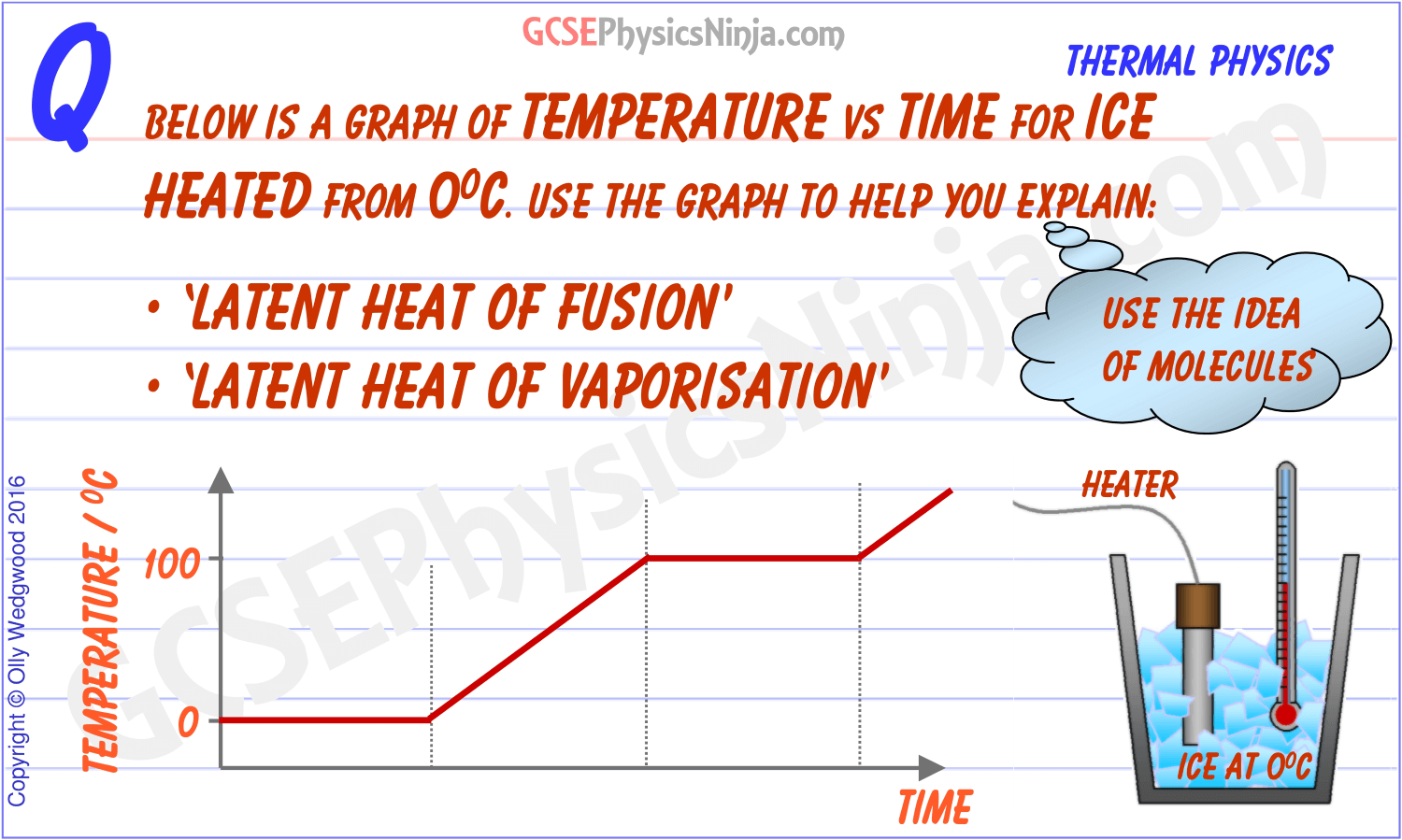
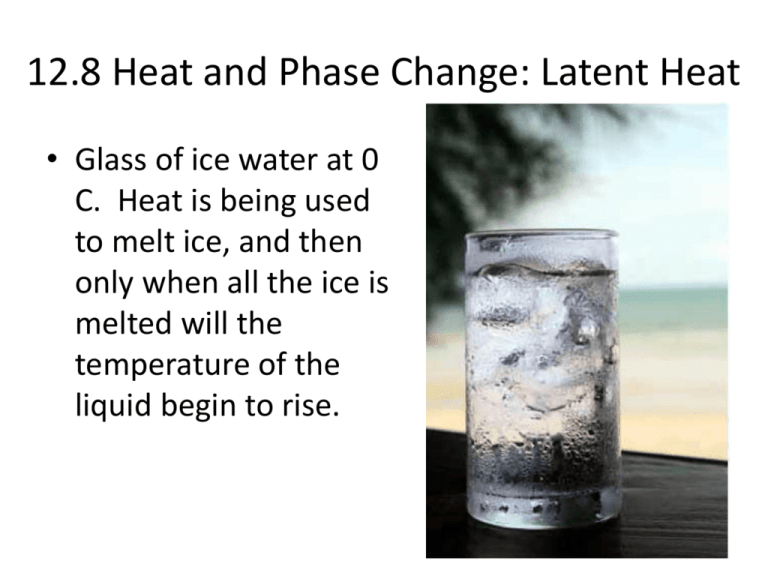
This energy doesn't immediately increase the temperature of the ice. Instead, it's used to disrupt the intermolecular forces holding the ice structure together.
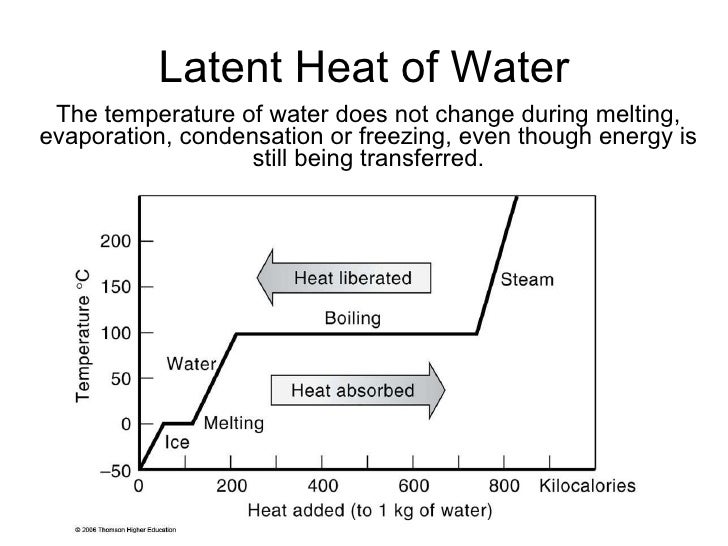
This is crucial: the temperature remains constant at 0°C (32°F) during the entire melting process. All the added heat is dedicated to breaking down the solid structure.
It's like trying to dismantle a Lego structure. You need to put in effort (energy) to pull the pieces apart, even if the individual pieces themselves don't get hotter.
Quantifying the Change
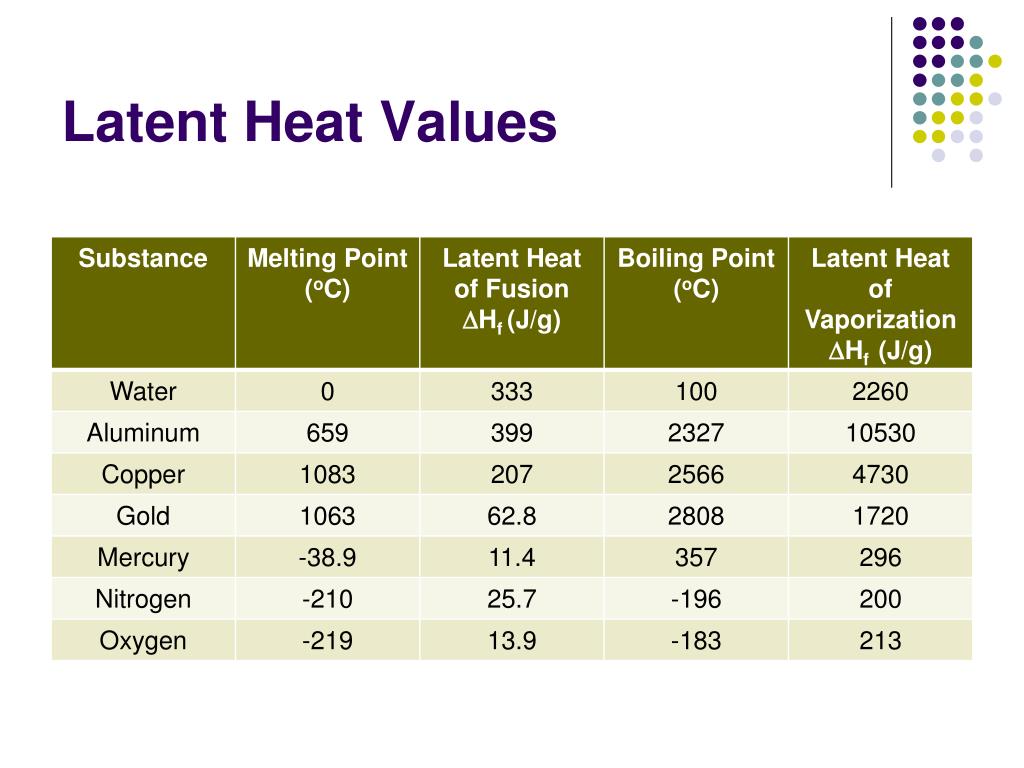
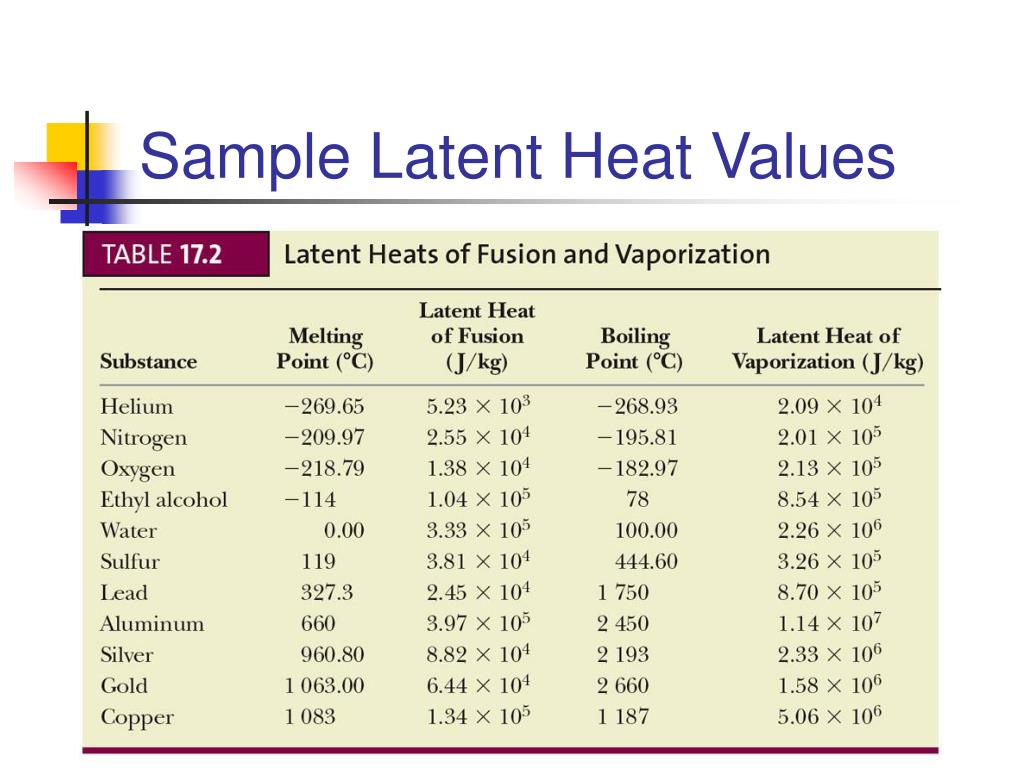
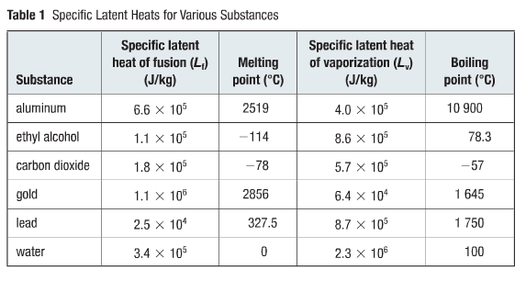
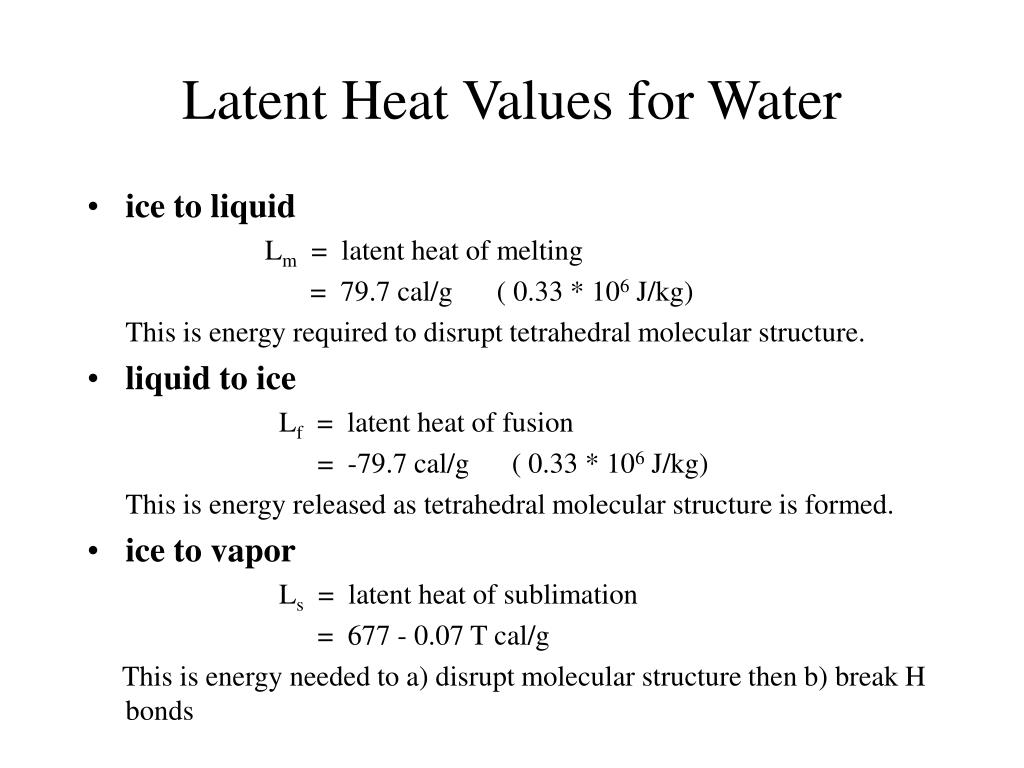
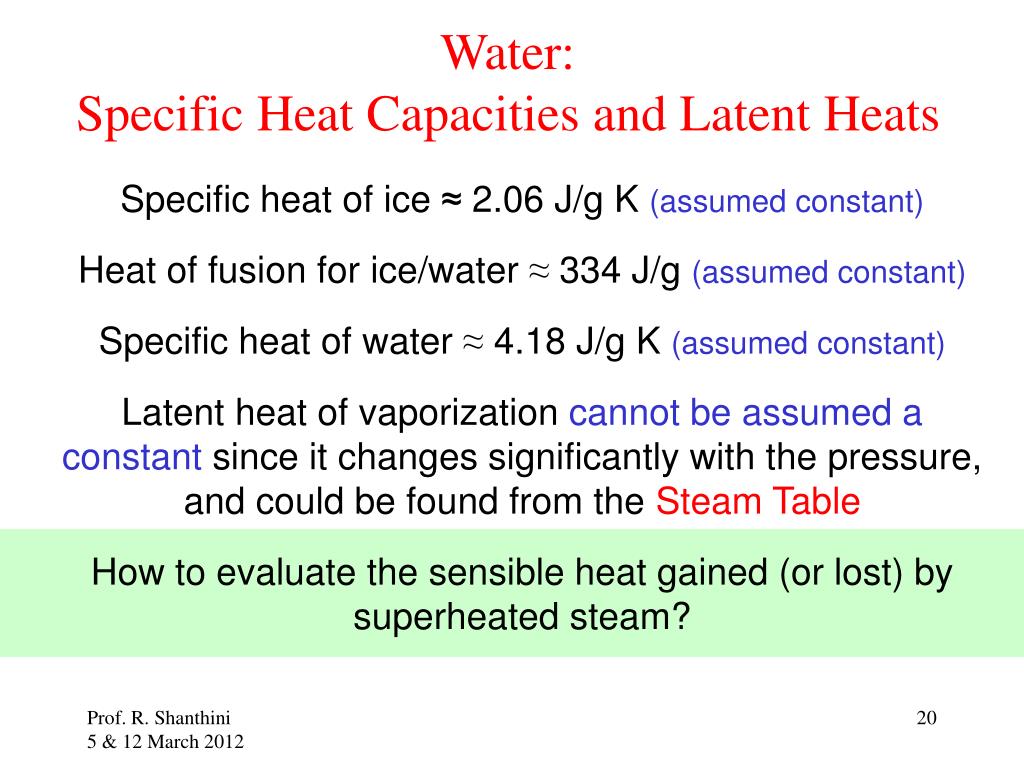
The latent heat of fusion for water is approximately 334 Joules per gram (J/g) or 80 calories per gram (cal/g). This means it takes 334 Joules of energy to melt one gram of ice at 0°C into water at 0°C.
That's a significant amount of energy! To put it in perspective, it takes much less energy to raise the temperature of one gram of water by 1°C.
This large value is why ice can have a powerful cooling effect – it absorbs a lot of heat as it melts.
The Role in Climate and Weather
Understanding latent heat is essential for comprehending various climate and weather phenomena.
For instance, the melting of ice in polar regions absorbs vast amounts of heat from the environment. This helps to regulate global temperatures.
Similarly, the evaporation of water (another phase change involving latent heat) cools the Earth's surface, while the condensation of water vapor releases heat into the atmosphere, driving weather patterns.
According to the National Oceanic and Atmospheric Administration (NOAA), "latent heat is a major player in Earth's energy budget and significantly affects climate." (This is a hypothetical NOAA statement for illustration purposes).
The energy absorbed or released during these phase changes influences air currents, cloud formation, and precipitation patterns.
Impact on Everyday Life
The principles of latent heat extend beyond the realm of scientific theory. They are applied in numerous practical applications.
Consider refrigeration systems: they utilize the latent heat of vaporization of refrigerants to absorb heat from the inside of a refrigerator.
This process cools the interior, keeping your food fresh. Another example is using ice packs to treat injuries. As the ice melts, it absorbs heat from the injured area, reducing swelling and pain.
Furthermore, understanding latent heat is crucial in designing efficient heating and cooling systems for buildings.
By incorporating materials that undergo phase changes at specific temperatures, engineers can create systems that store and release heat, reducing energy consumption and improving comfort.
Beyond the Classroom: The Ongoing Research
The study of latent heat continues to be an active area of research. Scientists are constantly seeking new ways to harness its potential for various applications.
For instance, research into phase-change materials (PCMs) is exploring novel materials that can efficiently store and release thermal energy.
These materials could revolutionize energy storage, waste heat recovery, and thermal management in electronic devices.
Moreover, a deeper understanding of the molecular dynamics during phase transitions can lead to the development of more accurate climate models.
Improved models enable more reliable predictions of future climate change impacts, allowing us to better prepare for and mitigate potential risks.
Reflections on a Simple Phenomenon
The next time you see ice melting, take a moment to appreciate the fascinating physics at play.
Latent heat, often overlooked, is a fundamental concept with profound implications for our planet and our daily lives.
It's a reminder that even the simplest phenomena can hold complex and beautiful truths, waiting to be uncovered and understood.



.jpg)