Optic Nerve Regeneration Clinical Trials 2025

The silent thief of sight, glaucoma, along with traumatic injuries and optic neuritis, leaves millions worldwide in a permanent darkness. But hope flickers on the horizon as 2025 dawns, carrying with it the promise of groundbreaking clinical trials focused on optic nerve regeneration. These trials, poised to launch at multiple sites across the globe, represent a pivotal moment in the quest to restore vision lost to optic nerve damage, offering a potential lifeline to those currently facing irreversible blindness.
At the core of this excitement lies a series of Phase I and Phase II clinical trials exploring diverse approaches to coaxing the damaged optic nerve into regrowth. These strategies range from gene therapy and stem cell transplantation to the use of neuroprotective agents and sophisticated bio-scaffolds. The collective goal: to bridge the gap in the severed nerve fibers and reconnect the eye to the brain, allowing visual information to once again flow.
Understanding the Challenge: Why Optic Nerve Regeneration is So Difficult
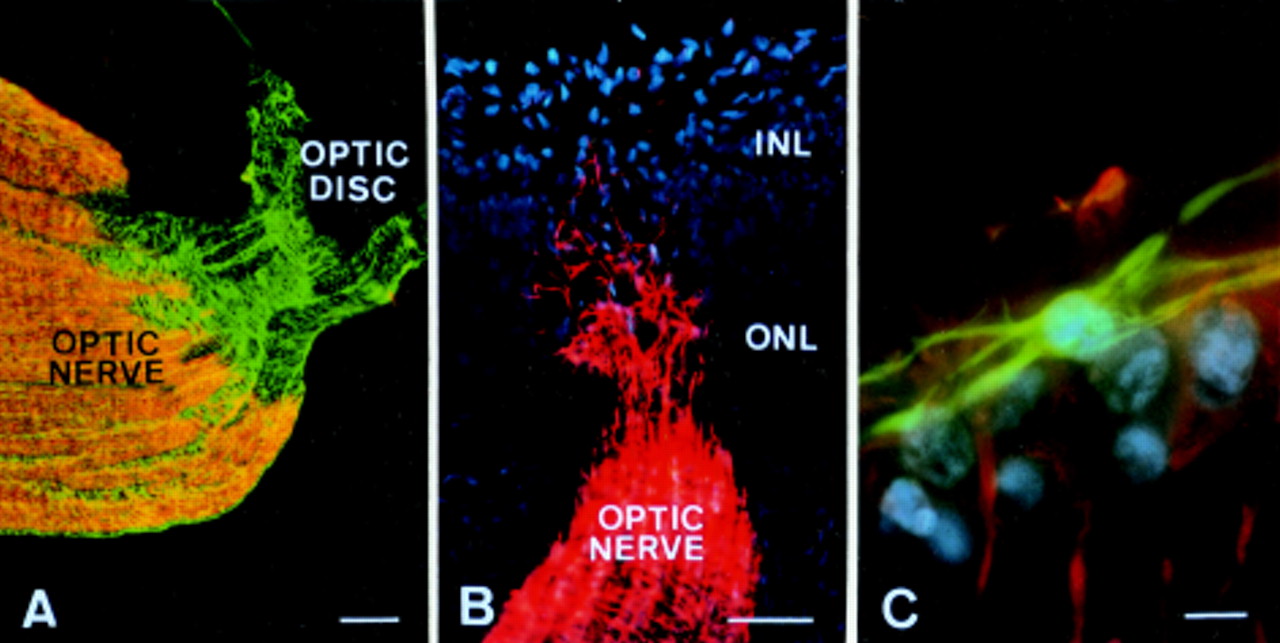
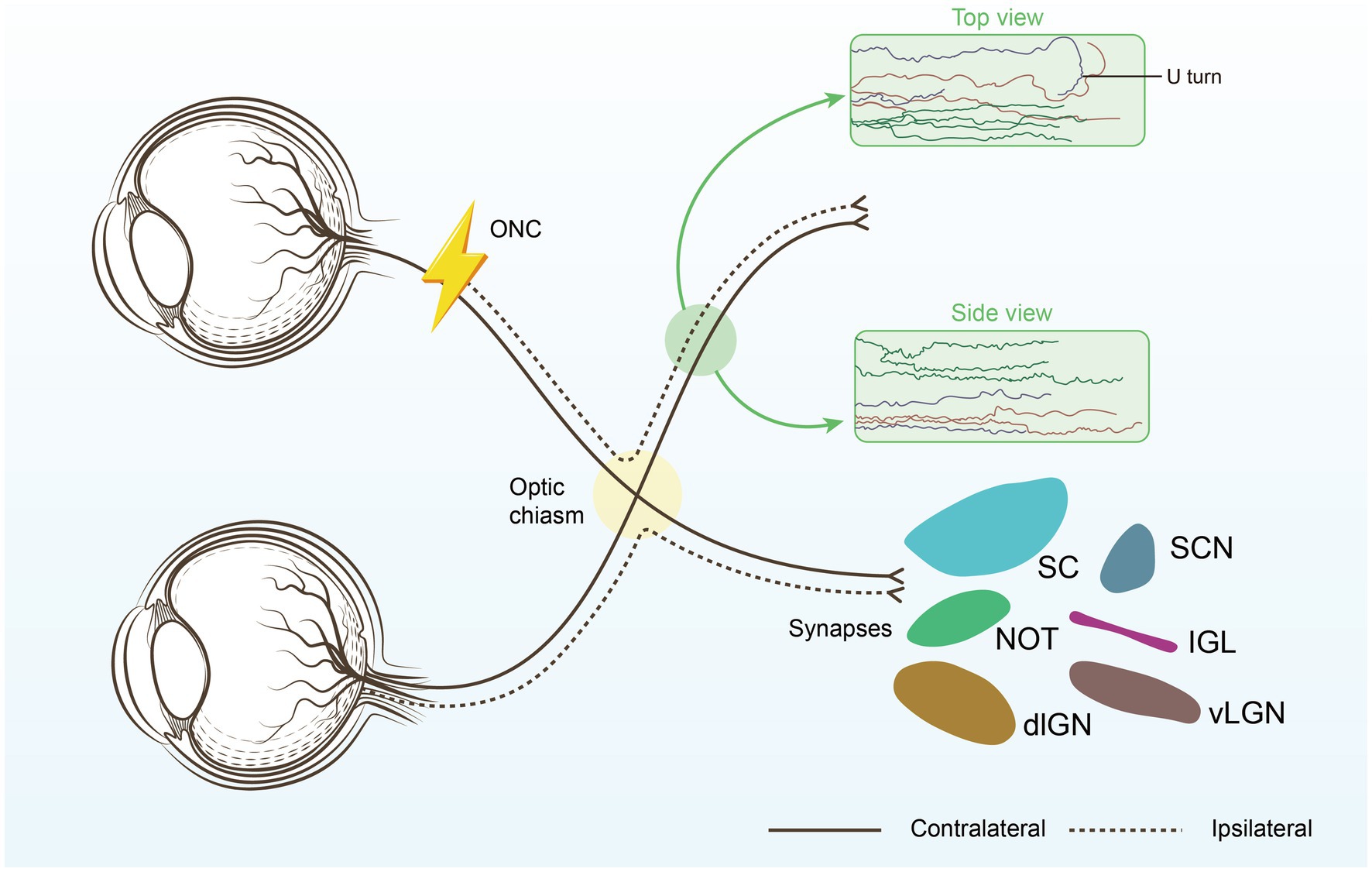
The optic nerve, unlike nerves in the peripheral nervous system, resides within the central nervous system (CNS). Regeneration in the CNS is notoriously difficult. This is primarily due to the presence of inhibitory molecules and a glial scar that forms at the site of injury.
These factors prevent axons, the long, slender projections of nerve cells that transmit electrical signals, from regrowing. Furthermore, even if axons do manage to sprout, navigating them back to their correct targets in the brain poses another significant hurdle.
The Leading Contenders: Clinical Trial Strategies
Several research groups are spearheading the 2025 clinical trials, each focusing on a unique strategy to overcome these regeneration barriers.
Gene Therapy Approaches
One prominent approach involves gene therapy. Researchers are using viral vectors to deliver genes that promote axon growth and block inhibitory signals into retinal ganglion cells (RGCs), the neurons that form the optic nerve. These trials, primarily led by teams at the University of California, San Diego and the University of Pennsylvania, aim to stimulate intrinsic regenerative capacity within the RGCs themselves.
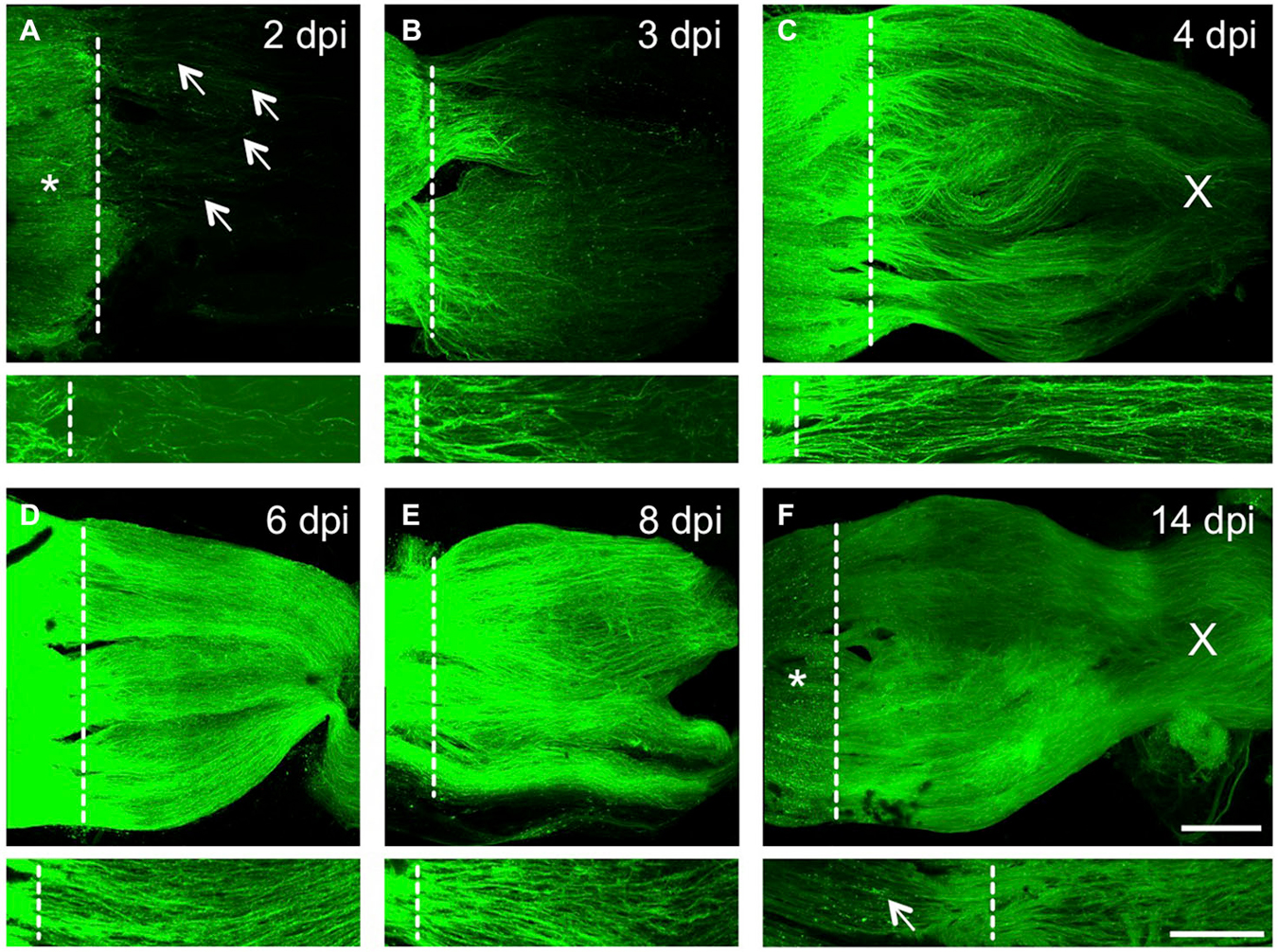
Preliminary pre-clinical data have shown encouraging results in animal models. These include increased axon regeneration and partial restoration of visual function.
Stem Cell Transplantation
Another promising avenue involves stem cell transplantation. This strategy focuses on replacing damaged RGCs with healthy, newly differentiated cells derived from stem cells. The National Eye Institute (NEI) is funding a multi-center trial using induced pluripotent stem cells (iPSCs) to generate RGCs for transplantation.
The transplanted cells are intended to integrate into the existing neural circuitry. Thus, it will re-establish the connection between the eye and the brain.
Neuroprotective Agents and Bio-Scaffolds
Beyond gene therapy and stem cells, research groups are exploring the use of neuroprotective agents to shield existing RGCs from further damage and promote their survival. Simultaneously, others are developing bio-scaffolds. These scaffolds are designed to provide a structural support system for regenerating axons, guiding them toward their targets in the brain.
A trial at Johns Hopkins University is combining neuroprotective agents with a bio-scaffold made of biodegradable materials. This is meant to create a conducive environment for nerve regeneration.
Patient Selection and Trial Design
The 2025 clinical trials will adhere to rigorous safety protocols and ethical guidelines. Patient selection criteria are stringent. They typically include individuals with recent optic nerve damage due to glaucoma, optic neuritis, or traumatic injury. The trials will primarily focus on assessing the safety and tolerability of the experimental treatments, with secondary endpoints evaluating potential improvements in visual function.
Visual acuity, visual field testing, and optical coherence tomography (OCT) will be used to monitor the health and function of the optic nerve.
"We are cautiously optimistic about the potential of these trials to provide a glimmer of hope for patients with irreversible vision loss,"commented Dr. Emily Carter, lead investigator of the gene therapy trial at the University of California, San Diego.
Ethical Considerations and Potential Challenges
While the promise of optic nerve regeneration is immense, ethical considerations are paramount. Informed consent is crucial. Patients need a clear understanding of the potential risks and benefits associated with these experimental therapies. Furthermore, the long-term effects of gene therapy and stem cell transplantation need careful monitoring.
Challenges remain, including the potential for immune rejection of transplanted cells, the difficulty of guiding regenerating axons to their correct targets, and the need for precise dosage and delivery of therapeutic agents.
A Glimmer of Hope: The Future of Vision Restoration
The 2025 clinical trials mark a significant step forward in the field of vision restoration. Even if these initial trials yield only modest improvements in visual function, they will provide invaluable data and pave the way for future advancements. The convergence of gene therapy, stem cell technology, and bioengineering offers unprecedented opportunities to tackle the challenges of optic nerve regeneration. The possibility of reversing blindness, once relegated to the realm of science fiction, is now inching closer to reality, bringing with it the hope of a brighter future for millions.