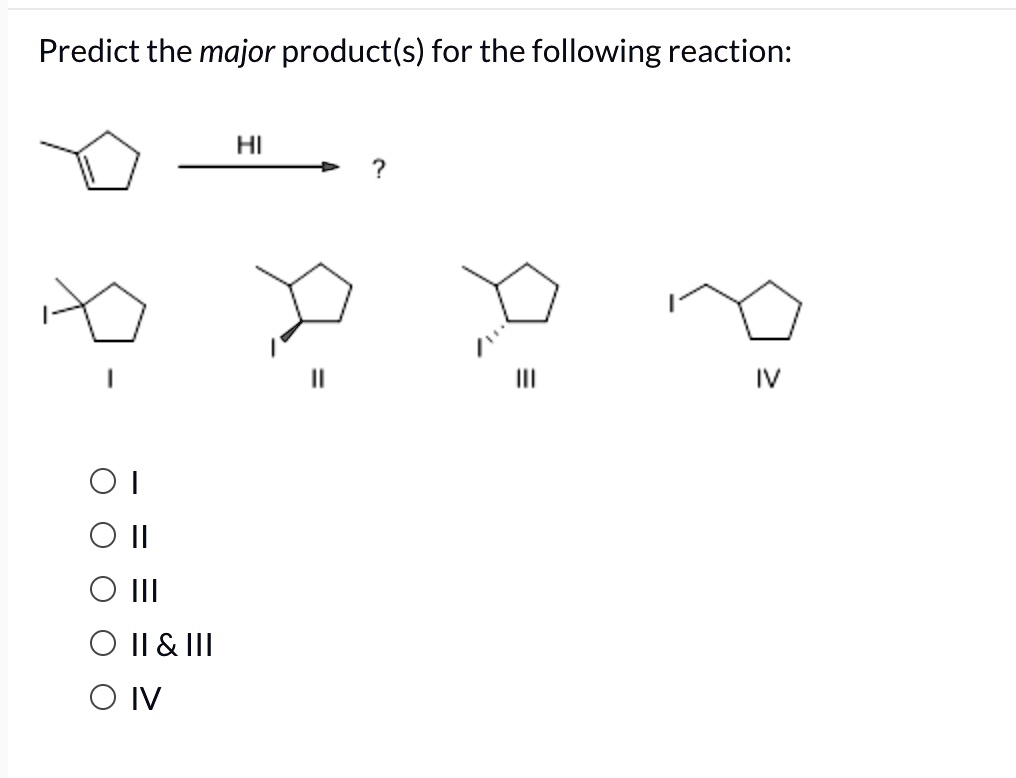
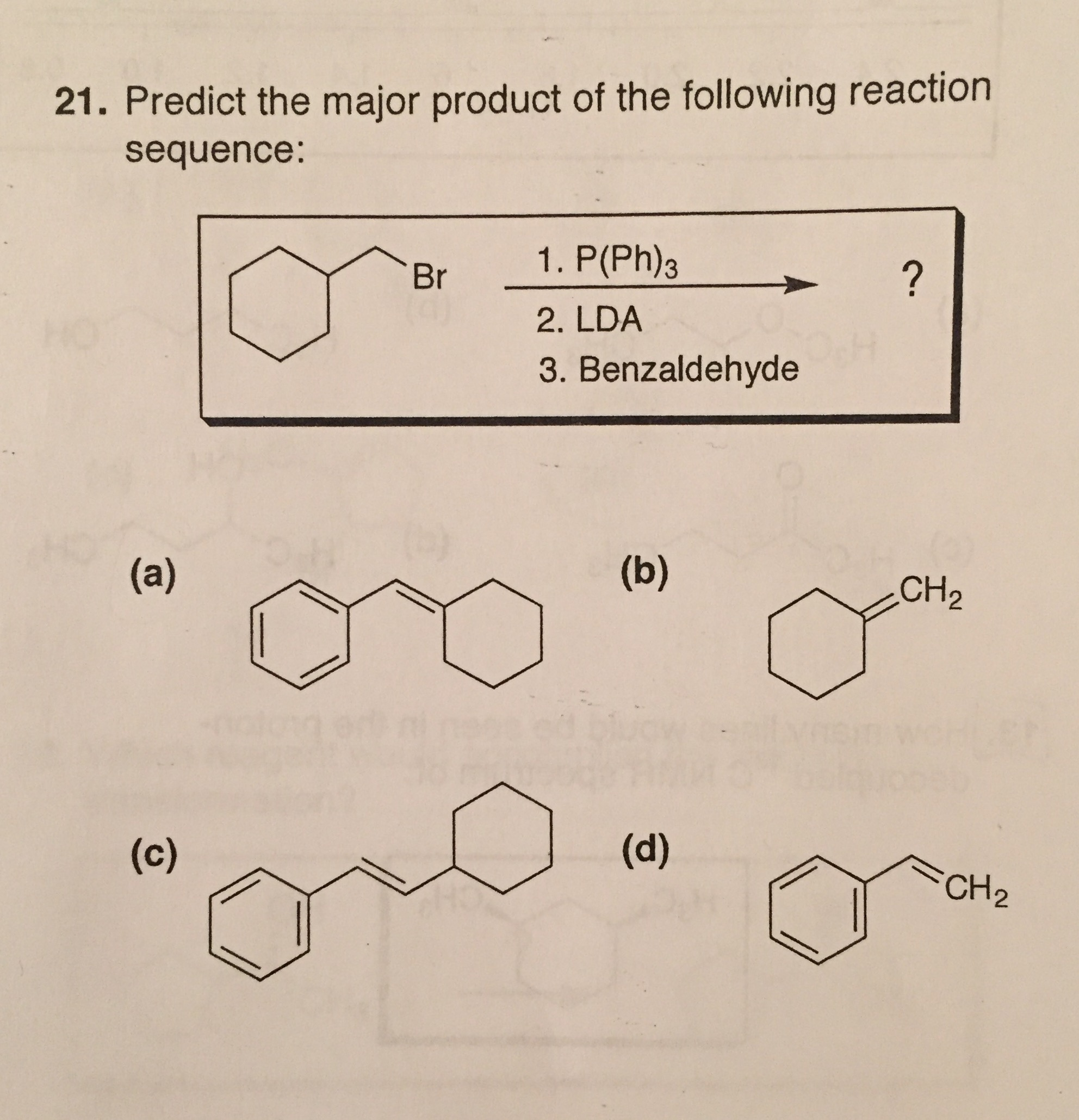
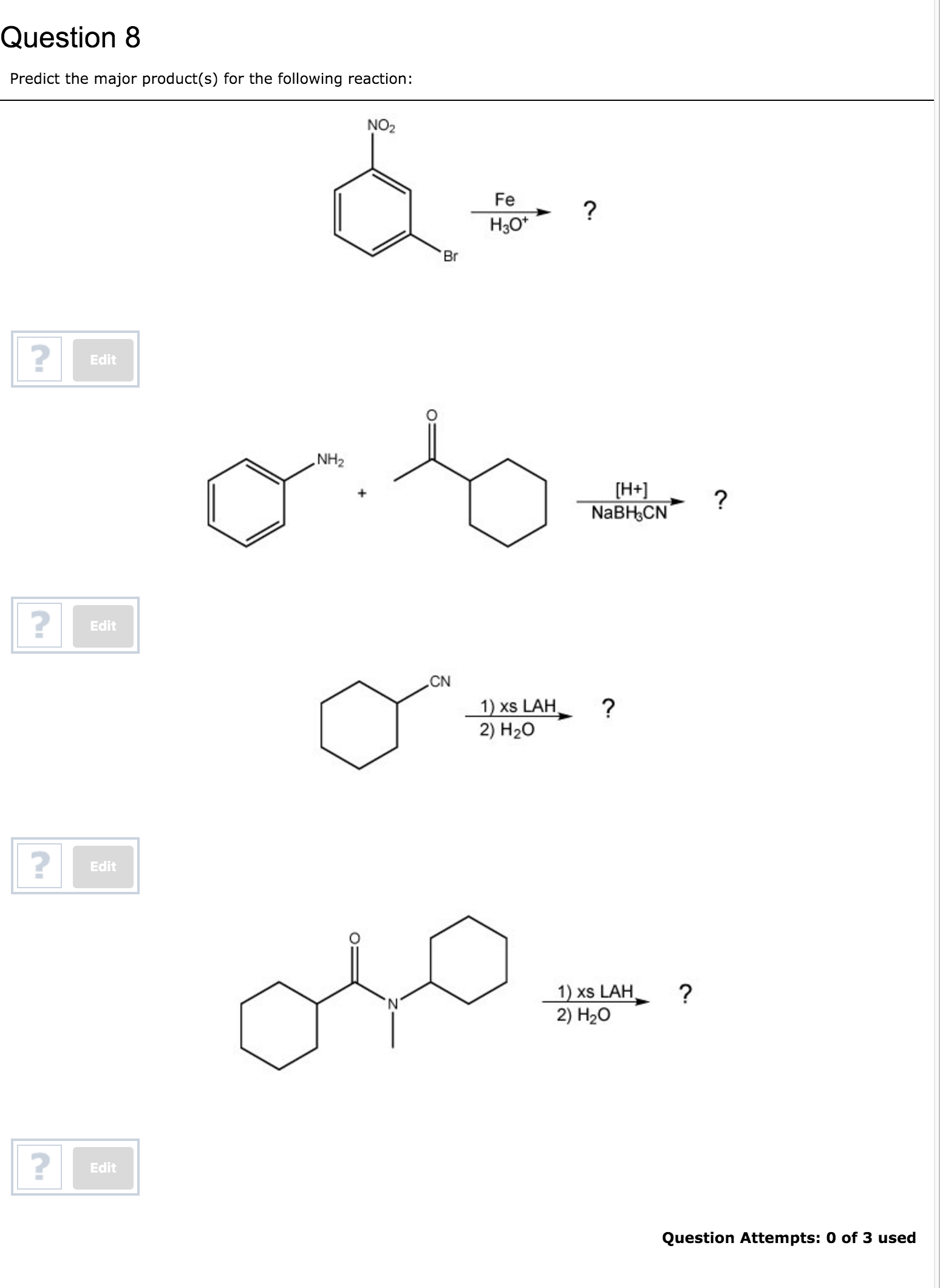
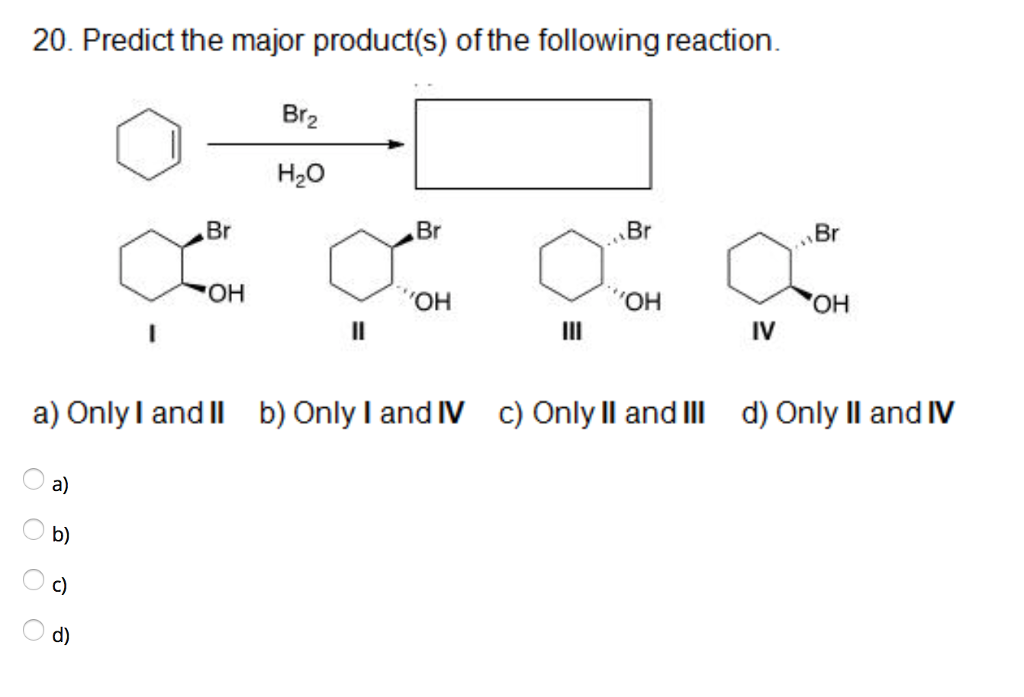
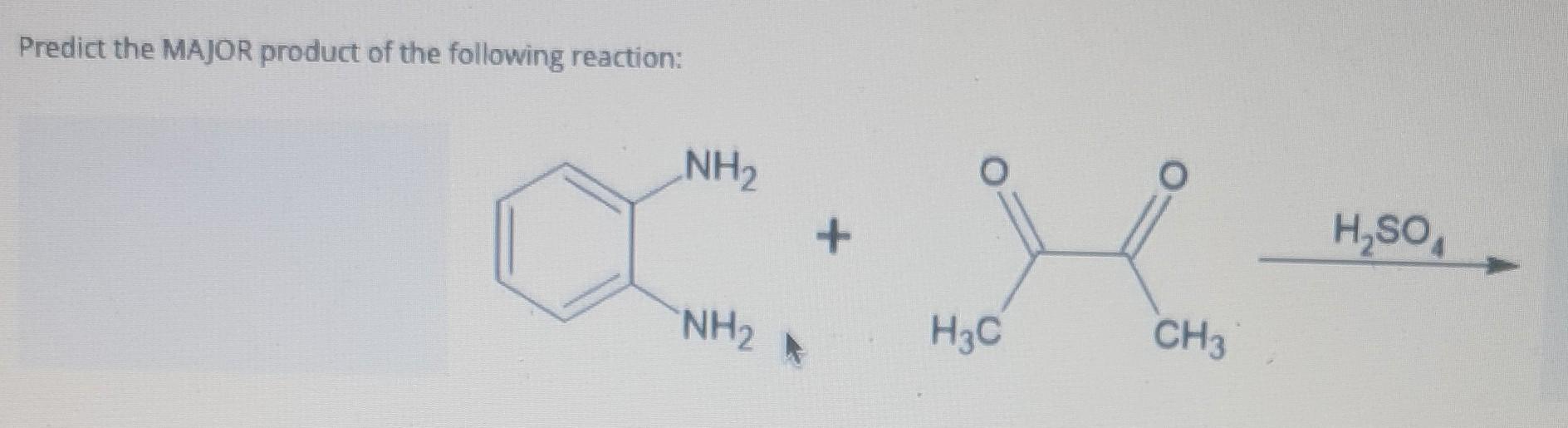
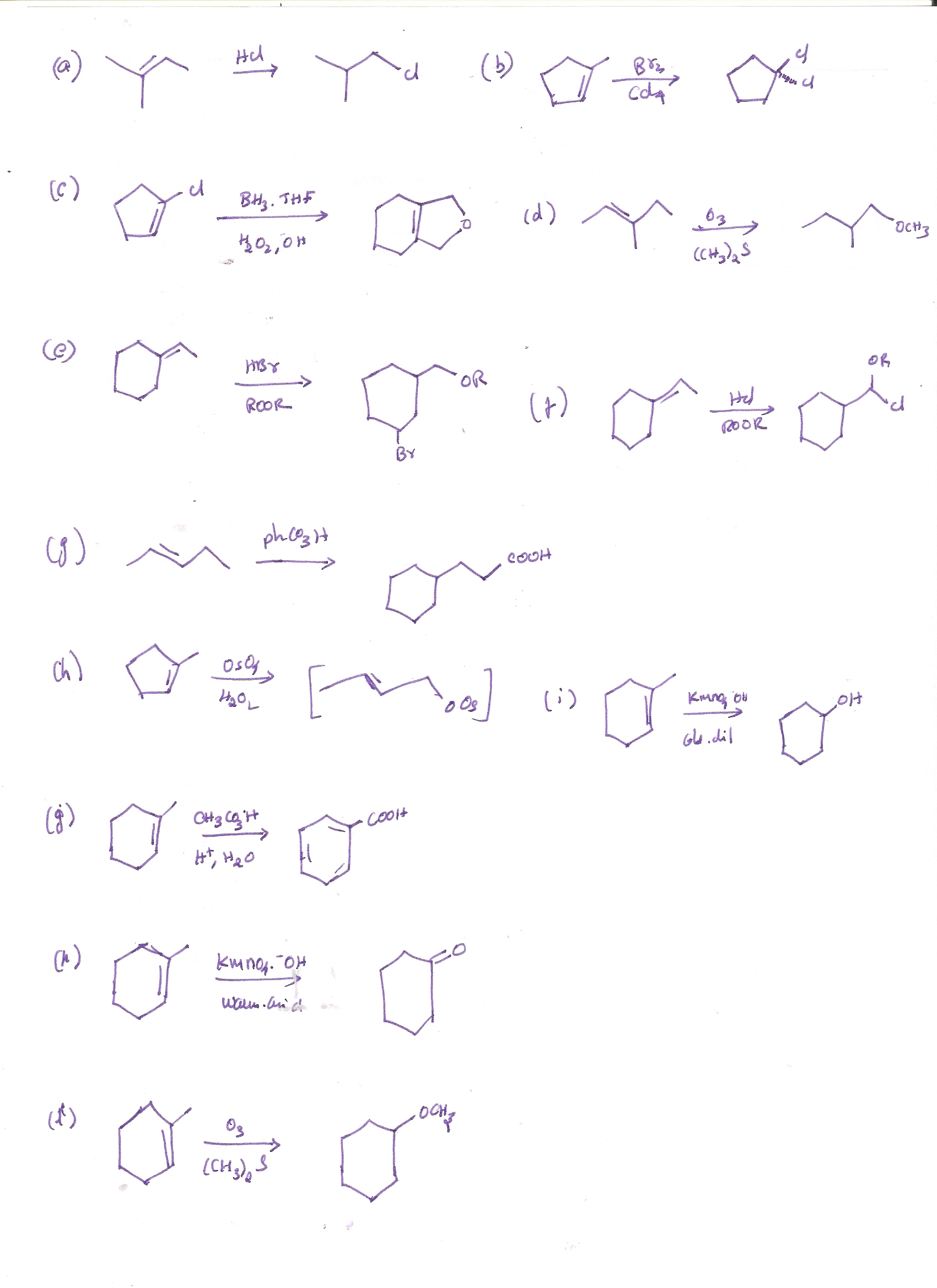Predict The Major Product S Of The Following Reaction
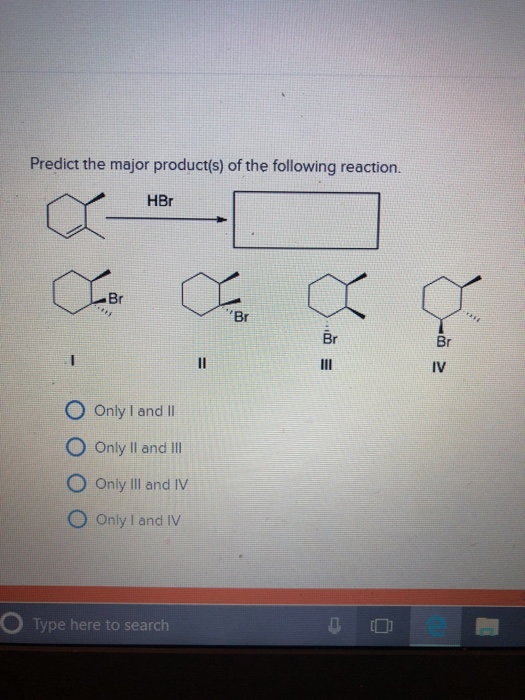
The seemingly innocuous query, "Predict the major product(s) of the following reaction," posed frequently in chemistry classrooms and online forums, has become a lightning rod for a deeper debate about the future of scientific education and the accessibility of complex knowledge.
The deceptively simple question, often accompanied by a chemical equation, masks a multifaceted challenge. It exposes vulnerabilities in students' understanding of fundamental chemical principles and also reflects the evolving landscape of scientific instruction in the digital age.
This article delves into the complexities surrounding this ubiquitous question. It examines the pedagogical implications and considers its role in shaping the next generation of scientists.
The Core Challenge: Mastering Organic Chemistry
At its heart, the "predict the product" question tests a student's grasp of organic chemistry principles. This includes reaction mechanisms, stereochemistry, and the influence of various functional groups.
Successfully answering requires a deep understanding of how molecules interact and transform under specific conditions. A lack of this understanding leads to errors, misconceptions and ultimately, an inability to solve more complex problems.
Professor Anya Sharma, a chemistry educator at the University of California, Berkeley, emphasizes the importance of mechanism comprehension: "Students who memorize reaction outcomes without understanding the underlying mechanisms are simply playing a guessing game. This won't lead to long-term retention or the ability to apply knowledge to novel situations."
The Digital Dilemma: Accessibility vs. Understanding
The internet has undeniably democratized access to information, including answers to chemistry problems. However, this accessibility creates a dilemma: students may rely on online resources for quick solutions instead of developing genuine understanding.
Platforms like Chegg and Course Hero offer step-by-step solutions to many textbook problems, including "predict the product" questions. This potentially hinders the development of critical thinking and problem-solving skills.
According to a recent study published in the Journal of Chemical Education, students who consistently utilize online resources for answers perform significantly worse on exams requiring application of knowledge. This reveals the crucial difference between passive information retrieval and active learning.
The Role of Visualization and Technology in Learning
Despite the potential pitfalls, technology can also be a powerful tool for enhancing chemistry education. Molecular visualization software and interactive simulations allow students to explore reaction mechanisms in a dynamic and engaging way.
These tools can help bridge the gap between abstract concepts and tangible representations. They make it easier to visualize the movement of electrons and the formation of new bonds.
Dr. Ben Carter, a developer of educational chemistry software, argues that: "The key is to use technology to supplement traditional teaching methods, not to replace them. We need to design tools that encourage exploration and critical thinking, rather than simply providing answers."
The Debate on Assessment Strategies
The "predict the product" question itself is a subject of debate among educators. Some argue that it is an overly simplistic assessment tool that fails to capture the depth of a student's understanding.
Alternative assessment methods, such as mechanism-based problem solving, require students to explicitly demonstrate their understanding of reaction pathways. These are seen as a more comprehensive and effective way to gauge learning.
Professor Sharma suggests incorporating more open-ended questions that require students to justify their answers and explain their reasoning. "This forces students to engage with the material in a more meaningful way and helps us identify areas where they may be struggling."
Addressing Misconceptions and Promoting Conceptual Understanding
Many common errors in predicting reaction products stem from fundamental misconceptions about chemical principles. These can include incorrect understanding of electrophilicity, nucleophilicity, and steric effects.
Targeted interventions, such as concept mapping and peer-led study groups, can help students address these misconceptions. They can reinforce their understanding of core concepts.
Dr. Carter emphasizes the importance of creating a supportive learning environment where students feel comfortable asking questions and admitting when they don't understand something. This involves fostering open communication and collaboration in the classroom.
The Future of Chemistry Education
The challenge of "predicting the product" reflects a larger need to adapt chemistry education to the digital age. This includes embracing technology in a responsible way. It also means fostering critical thinking, and promoting conceptual understanding over rote memorization.
By incorporating innovative teaching strategies, utilizing technology effectively, and emphasizing mechanism-based problem solving, educators can equip students with the skills and knowledge they need to succeed in the ever-evolving field of chemistry. This will ensure that the next generation of scientists is well-prepared to tackle the complex challenges facing our world.
The ongoing conversation about "predict the product" will hopefully lead to a more nuanced and effective approach to chemistry education. It will equip students with a deeper understanding of chemistry principles.