The Resting Membrane Potential Results From

The intricate dance of ions across a cell's membrane, a process occurring constantly within our bodies, underlies a fundamental principle of life: the resting membrane potential. This electrical potential difference, vital for nerve impulse transmission, muscle contraction, and cellular communication, is not a static state but a carefully maintained balance driven by a confluence of factors.
Understanding the mechanics behind this crucial potential is paramount for advancements in treating neurological disorders, cardiac arrhythmias, and a host of other conditions. Scientists continue to unravel the precise contributions of each component, paving the way for targeted therapies.
The Foundation: Ion Concentrations
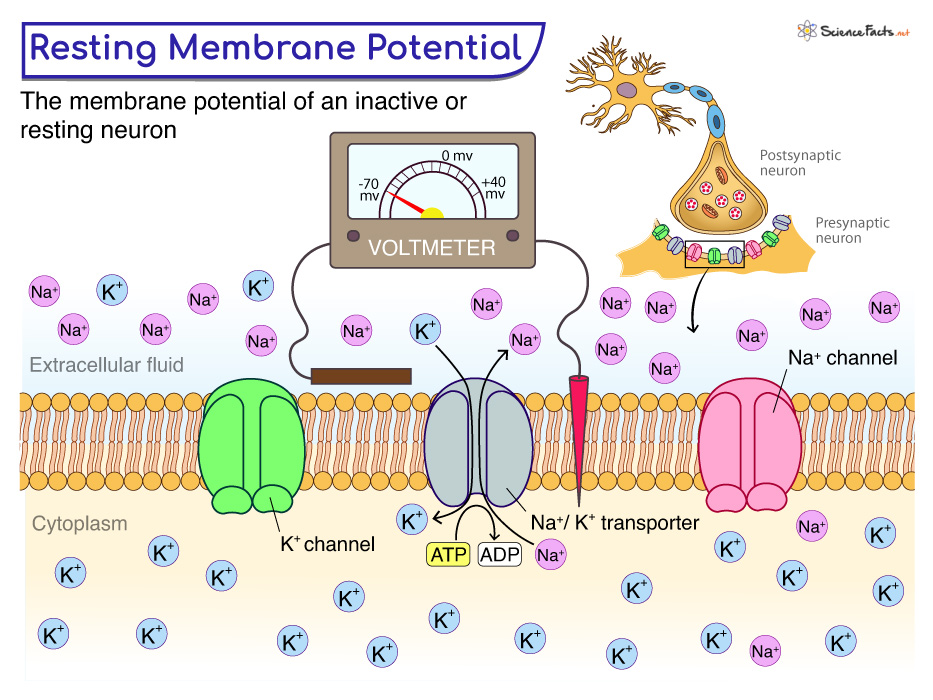
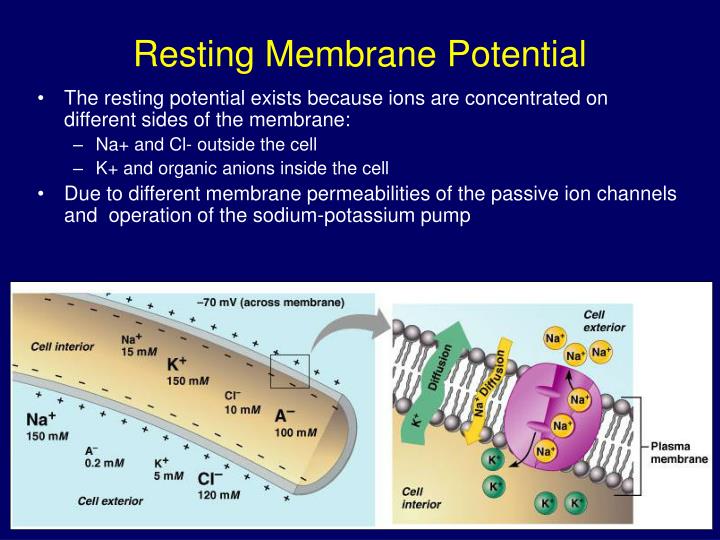
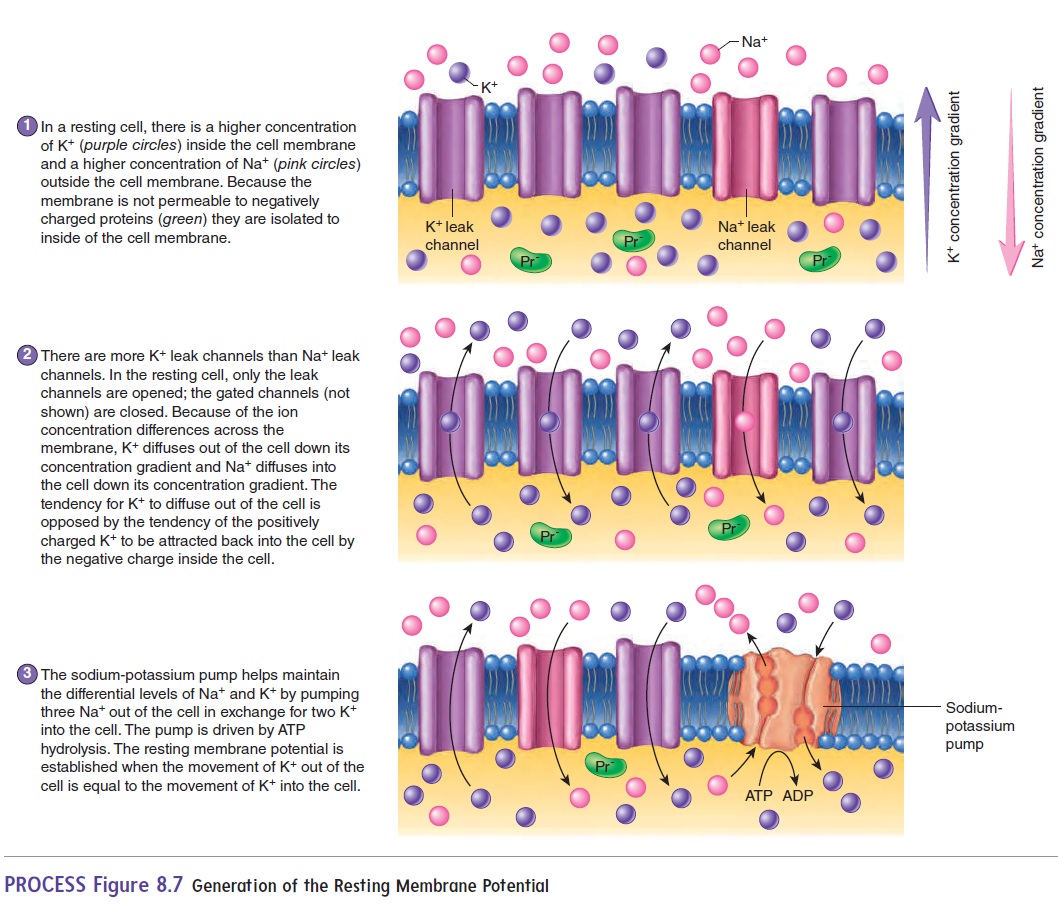
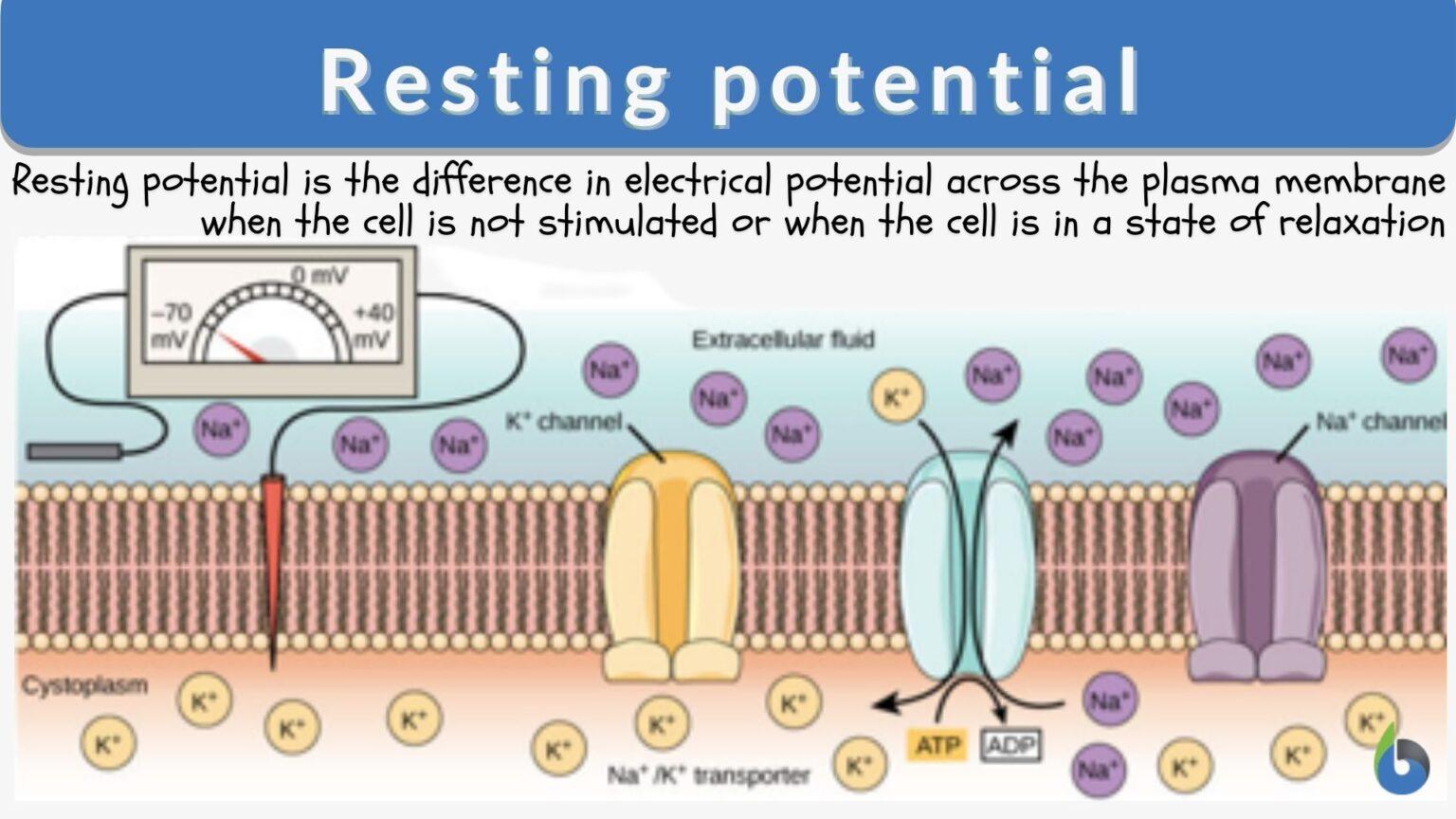
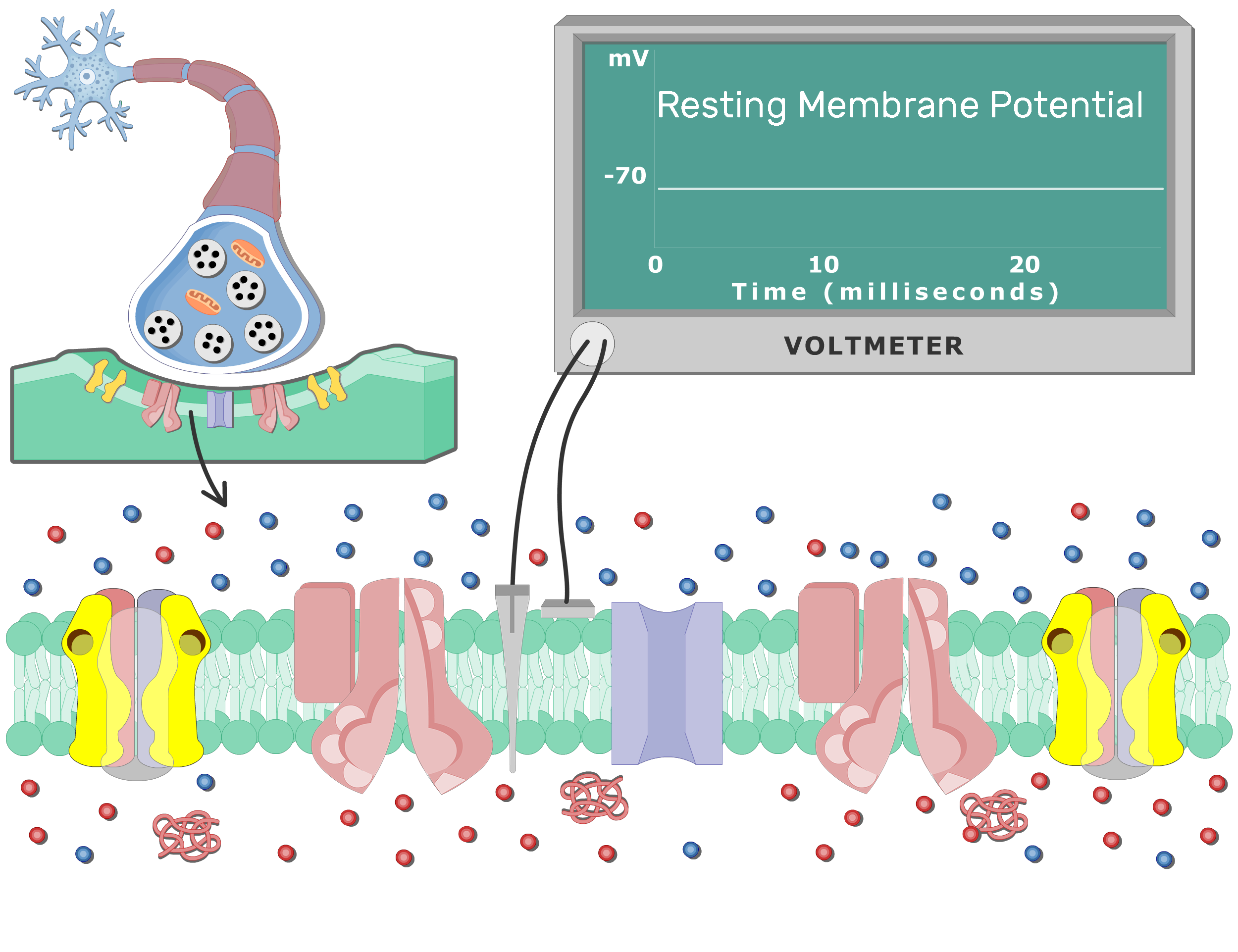
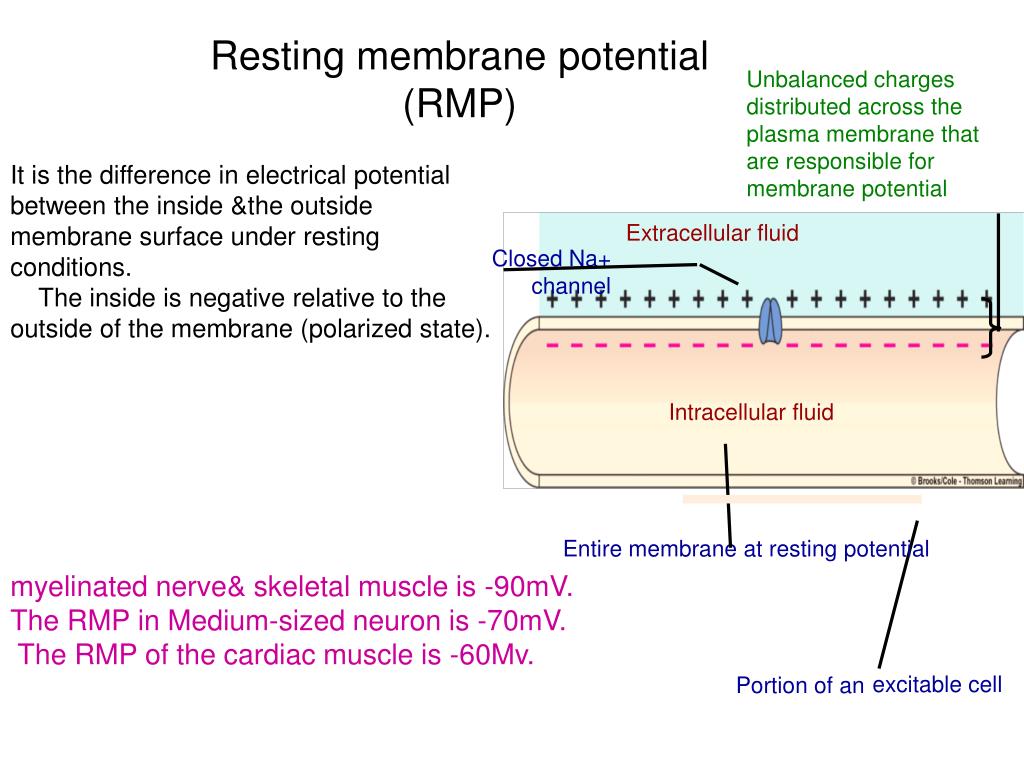
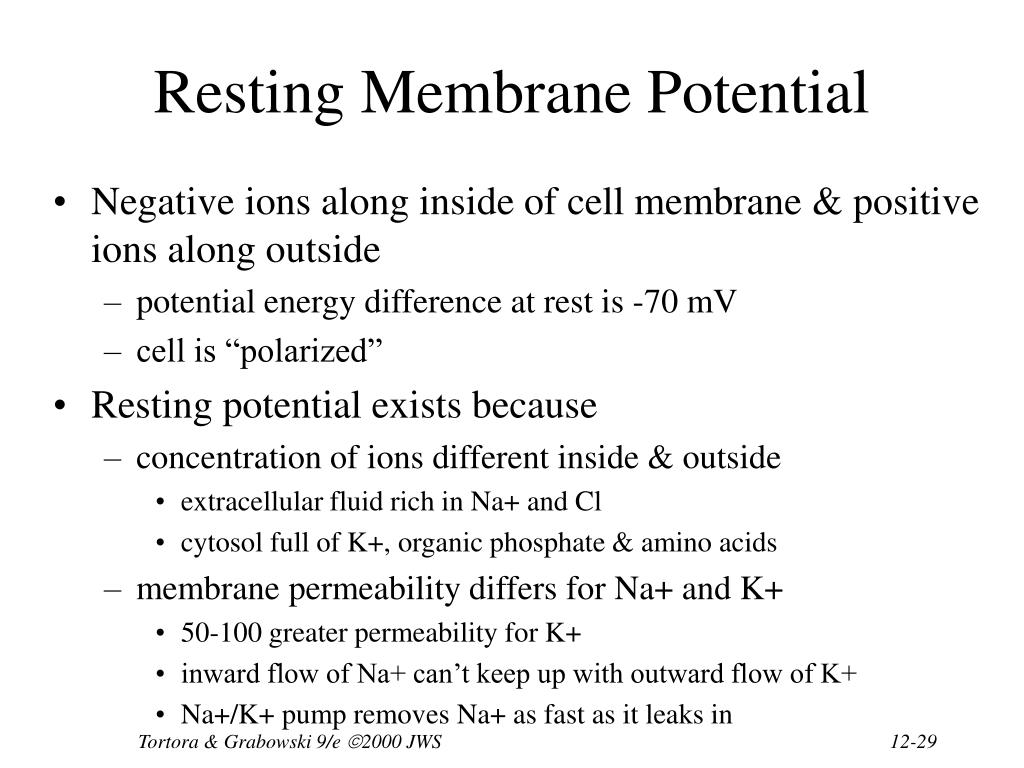
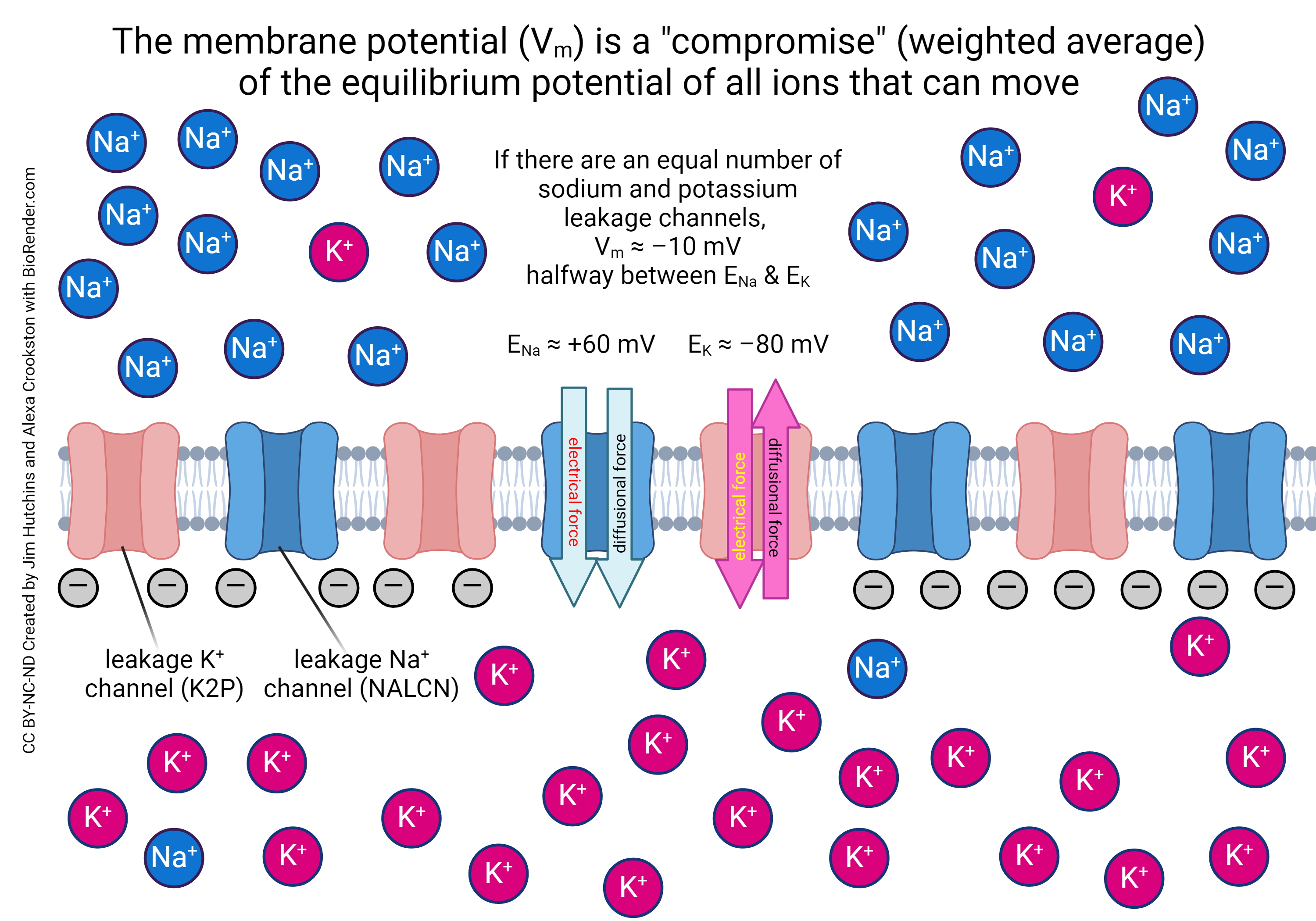
The resting membrane potential is primarily established by the unequal distribution of ions, specifically sodium (Na+), potassium (K+), chloride (Cl-), and negatively charged proteins (A-), across the cell membrane. This difference in concentration creates a chemical driving force, urging ions to move from areas of high concentration to areas of low concentration.
Inside the cell, potassium (K+) concentration is significantly higher, while sodium (Na+) and chloride (Cl-) concentrations are lower compared to the extracellular fluid. These gradients are not spontaneous; they are actively maintained by specialized proteins in the cell membrane.
Key Player: The Sodium-Potassium Pump
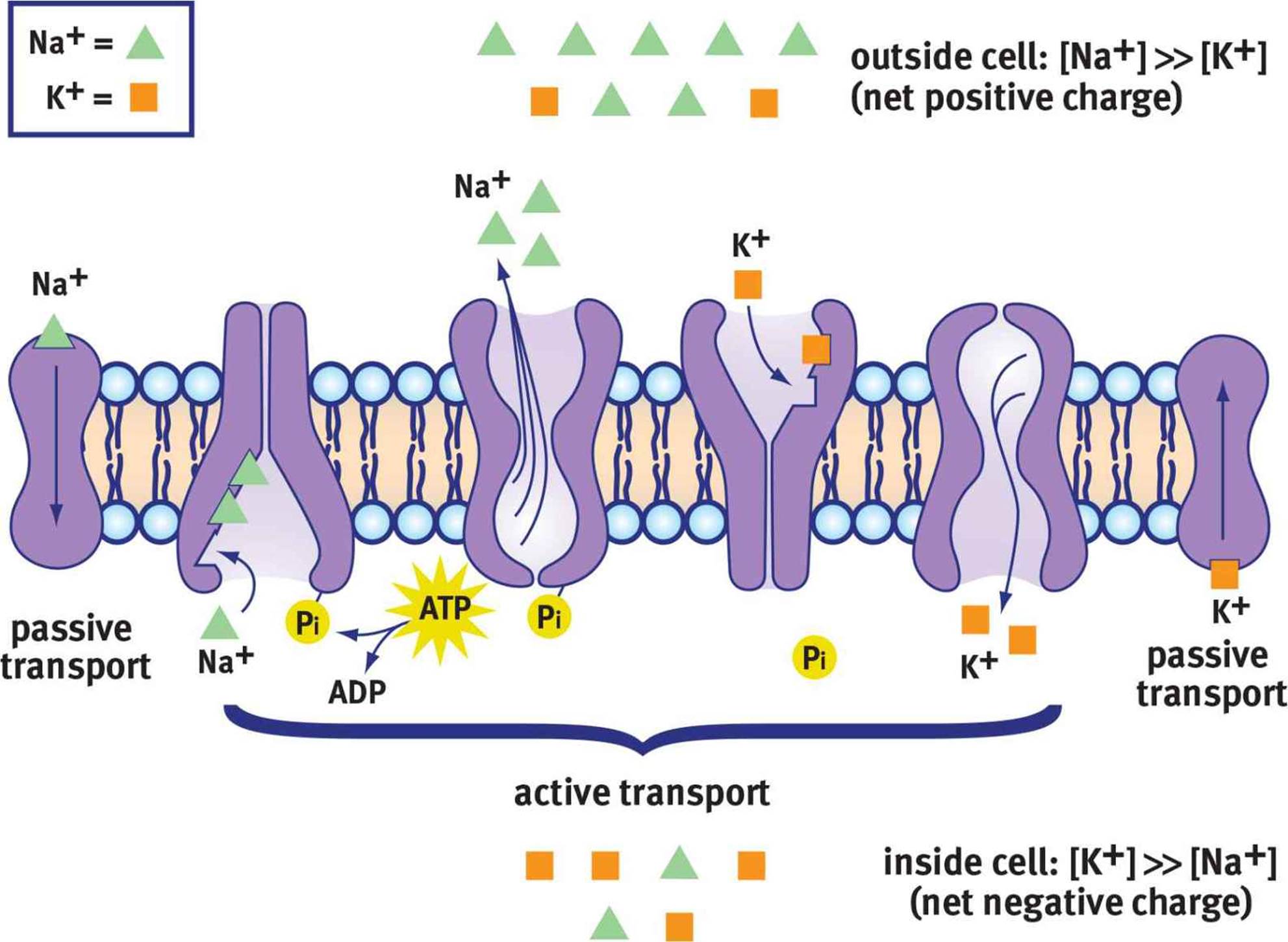
The sodium-potassium pump, also known as Na+/K+ ATPase, is a transmembrane protein that actively transports three sodium ions (Na+) out of the cell and two potassium ions (K+) into the cell. This process requires energy in the form of ATP (adenosine triphosphate), hence it's considered active transport.
The pump works against the electrochemical gradients of both ions, contributing to the negative charge inside the cell. Its ongoing activity is essential for maintaining the proper ion concentrations and, consequently, the resting membrane potential.
Ion Channels: Gateways for Selective Permeability
While the sodium-potassium pump establishes the concentration gradients, ion channels determine the membrane's permeability to different ions. These protein structures span the cell membrane, forming pores that allow specific ions to pass through based on their size and charge.
At rest, the membrane is significantly more permeable to potassium (K+) than to sodium (Na+). This selective permeability means that potassium ions can leak out of the cell down their concentration gradient, contributing to the negative charge inside. This is a critical aspect of establishing the resting membrane potential.
Equilibrium Potential: The Nernst Equation
The Nernst equation is a mathematical formula used to calculate the equilibrium potential for a specific ion. This potential represents the membrane potential at which the electrical driving force on an ion is equal and opposite to the chemical driving force, resulting in no net movement of the ion across the membrane.
For potassium (K+), the Nernst potential is typically around -90 mV, meaning that at this potential, there's no net movement of K+ across the membrane. Sodium's (Na+) Nernst potential is around +60mV.
Goldman-Hodgkin-Katz (GHK) Equation: Weighing the Influences
The Goldman-Hodgkin-Katz (GHK) equation is an extension of the Nernst equation that takes into account the permeability of the membrane to multiple ions. It calculates the resting membrane potential based on the concentration gradients and relative permeability of all relevant ions, typically sodium (Na+), potassium (K+), and chloride (Cl-).
Because the membrane is more permeable to potassium at rest, the resting membrane potential is closer to the Nernst potential for potassium than for sodium. The typical resting membrane potential for a neuron is around -70 mV.
The Role of Chloride Ions
Chloride ions (Cl-) also contribute to the resting membrane potential, although their role is often less prominent than that of sodium and potassium in many cell types. Their distribution and permeability can vary depending on the cell type and physiological conditions.
In some neurons, chloride channels are open at rest, allowing Cl- to move across the membrane and influence the resting potential. In other cell types, the contribution of chloride is minimal.
Clinical Significance and Future Directions
Disruptions in the resting membrane potential can have profound consequences for cellular function and overall health. For example, alterations in ion channel activity can lead to neurological disorders such as epilepsy, while abnormalities in cardiac ion channels can cause arrhythmias.
Researchers are actively investigating the mechanisms that regulate ion channel function and the factors that contribute to the maintenance of the resting membrane potential.
Understanding these processes is crucial for developing targeted therapies for a wide range of diseases.
Further research into the complexities of ion channel regulation and the interplay between different ion gradients promises to unlock new avenues for treating diseases linked to imbalances in the resting membrane potential. This knowledge will be vital in developing precise therapies that address the root causes of these conditions.