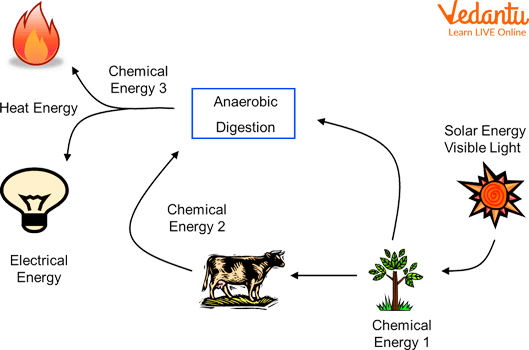
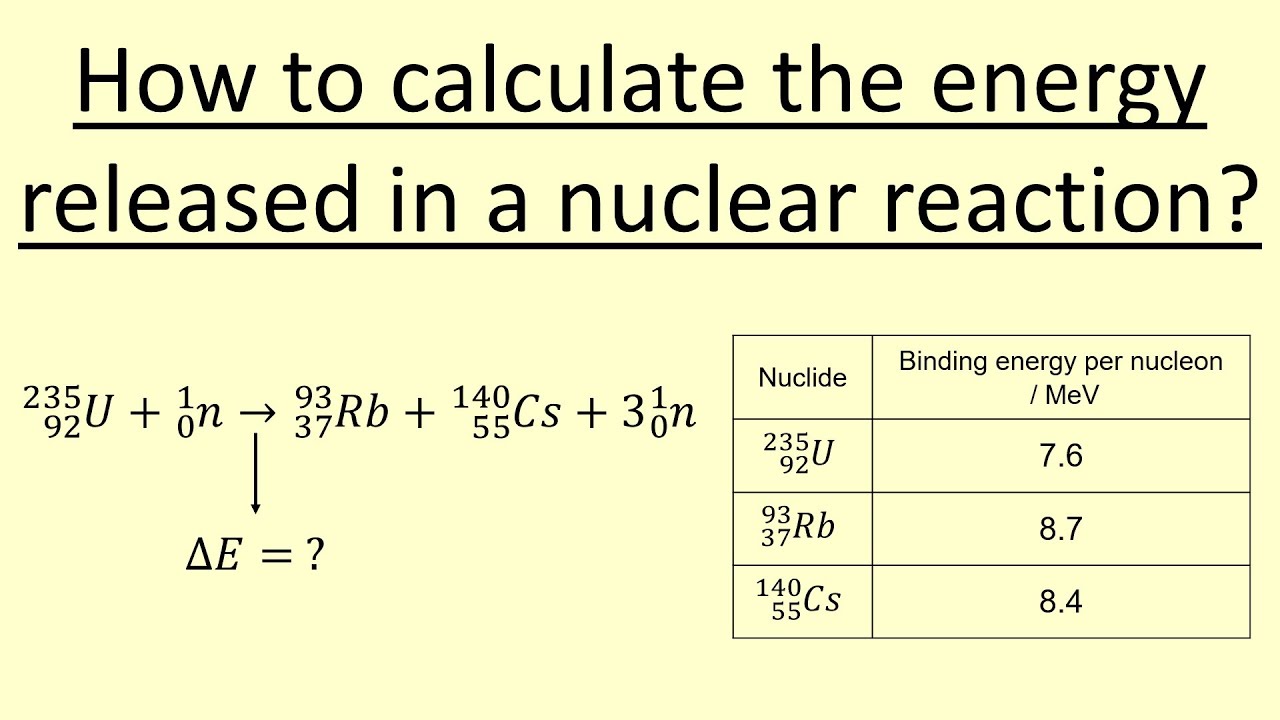
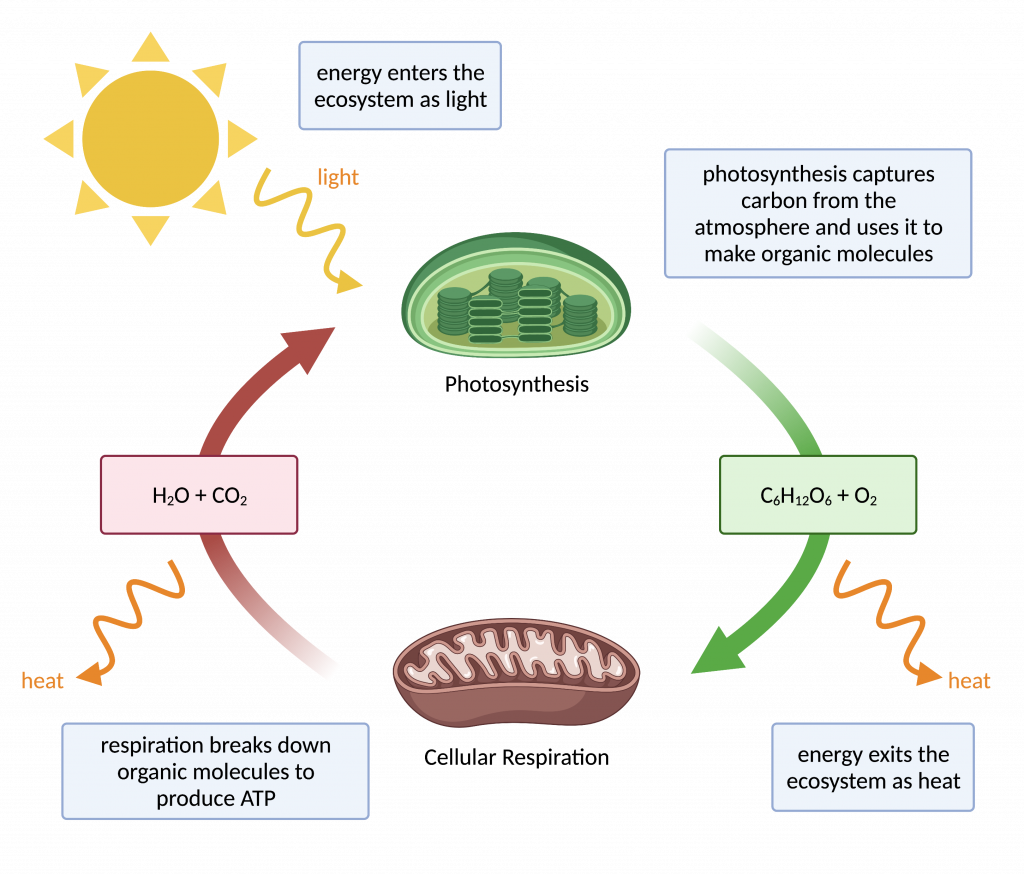
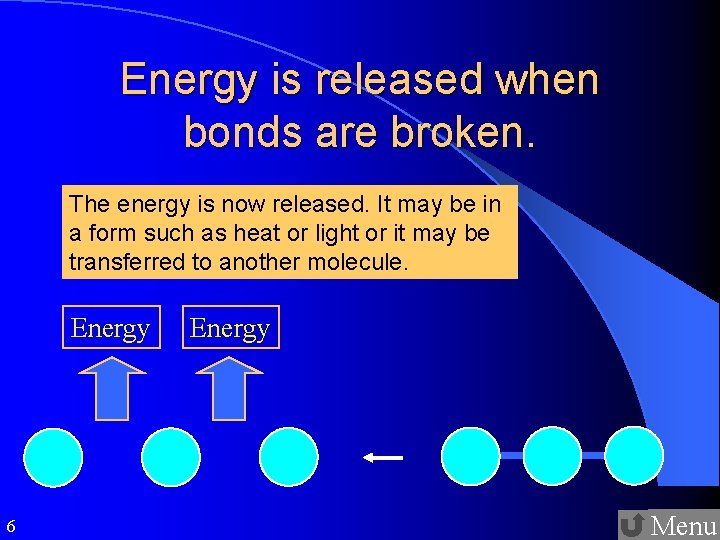
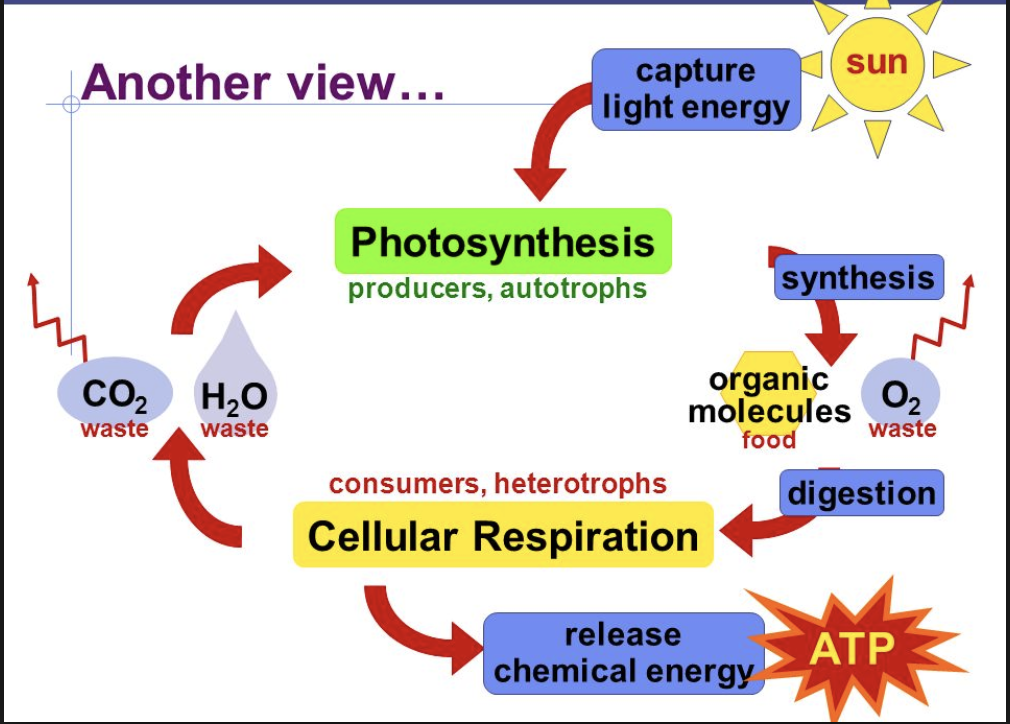
Through Which Conversion Is Energy Released

The question of how energy is released is fundamental to our understanding of the universe, underpinning everything from the sun's radiant heat to the controlled reactions powering nuclear plants. This process, at its core, involves the conversion of one form of energy into another, often accompanied by the release of energy as heat, light, or other forms of radiation. Recent research and ongoing advancements in fields like nuclear physics and thermodynamics continue to refine our knowledge of these intricate energy transformations.
Understanding the mechanisms through which energy is released is critical for addressing global challenges such as energy production, climate change mitigation, and technological advancements. This understanding is not merely an academic exercise; it directly influences how we power our world, develop new technologies, and comprehend the fundamental laws governing nature. This article explores the primary energy conversion processes that result in energy release, outlining the scientific principles involved and highlighting their significance.
Nuclear Fusion and Fission
One of the most potent forms of energy release occurs through nuclear reactions, specifically fusion and fission. These processes involve the manipulation of atomic nuclei, releasing tremendous amounts of energy according to Einstein's famous equation, E=mc². This equation demonstrates the equivalence of mass and energy, indicating that a small amount of mass can be converted into a substantial quantity of energy.
Nuclear fusion, the process that powers the sun and other stars, involves combining light atomic nuclei, such as hydrogen isotopes, to form heavier nuclei like helium. This fusion reaction releases a significant amount of energy because the mass of the resulting nucleus is slightly less than the sum of the masses of the original nuclei. This 'missing' mass is converted into energy according to E=mc².
In contrast, nuclear fission involves the splitting of a heavy atomic nucleus, such as uranium or plutonium, into two or more smaller nuclei. This process is typically initiated by bombarding the heavy nucleus with a neutron. The fission process releases energy, additional neutrons, and other particles, leading to a chain reaction that can be controlled in nuclear reactors to generate electricity or uncontrolled in nuclear weapons.
Chemical Reactions
Chemical reactions also release energy through the breaking and forming of chemical bonds between atoms and molecules. These reactions are governed by the principles of thermodynamics, which describe the relationships between heat, work, and energy.
Exothermic reactions are chemical processes that release energy in the form of heat. Combustion, the burning of fuels such as wood, propane, or gasoline, is a common example of an exothermic reaction. During combustion, chemical bonds in the fuel molecules are broken, and new bonds are formed with oxygen atoms, releasing energy as heat and light.
The energy released in exothermic reactions is often measured in terms of enthalpy, a thermodynamic property representing the total heat content of a system. A negative change in enthalpy indicates that energy is released from the system to the surroundings.
Thermodynamic Processes
Beyond chemical reactions, energy is also released through various thermodynamic processes involving changes in temperature, pressure, and volume of a system. These processes are governed by the laws of thermodynamics, particularly the first and second laws.
The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, only converted from one form to another. This principle implies that any energy released in a thermodynamic process must come from a corresponding decrease in the internal energy of the system.
The second law of thermodynamics introduces the concept of entropy, a measure of the disorder or randomness of a system. According to this law, energy conversions are never perfectly efficient, and some energy is always lost as heat due to increasing entropy. For example, in a heat engine, only a fraction of the heat input is converted into useful work, with the remainder dissipated as waste heat.
Applications and Implications
The understanding of these energy conversion processes has profound implications for various fields, including energy production, technology, and environmental science. The development of more efficient energy conversion technologies is crucial for reducing our reliance on fossil fuels and mitigating climate change.
For instance, research into advanced nuclear reactor designs aims to improve the efficiency and safety of nuclear power generation. Similarly, efforts to develop renewable energy sources, such as solar and wind power, focus on optimizing the conversion of sunlight and wind into electricity.
Furthermore, a deeper understanding of thermodynamic principles is essential for designing more efficient engines, power plants, and industrial processes. By minimizing energy losses and maximizing energy recovery, we can reduce our overall energy consumption and environmental impact.
"Continued research and development in these areas are critical for ensuring a sustainable and prosperous future," according to a recent statement from the Department of Energy.
Conclusion
The release of energy is a fundamental process that occurs through various mechanisms, including nuclear reactions, chemical reactions, and thermodynamic processes. Each of these processes involves the conversion of one form of energy into another, often with the release of energy as heat, light, or other forms of radiation. Our understanding of these energy conversions is constantly evolving, driven by ongoing research and technological advancements.
By continuing to explore and refine our knowledge of energy release, we can develop more efficient energy technologies, address global challenges such as climate change, and unlock new possibilities for technological innovation. The quest to understand and harness energy remains one of the most important scientific and technological endeavors of our time.