Under What Circumstances Can An Atom Emit A Photon
Imagine a firefly, blinking its tiny light in the velvet darkness of a summer night. That brief flash, a miniature beacon in the vast expanse, is a reminder that even the smallest things can emit light. But how does it happen? What compels these creatures, and more importantly, atoms, to release that energy in the form of a photon?
At the heart of this phenomenon lies the intricate world of quantum mechanics. This article delves into the fascinating circumstances under which an atom can emit a photon, exploring the fundamental principles that govern this process and its profound implications for our understanding of the universe.
The Atom's Inner World
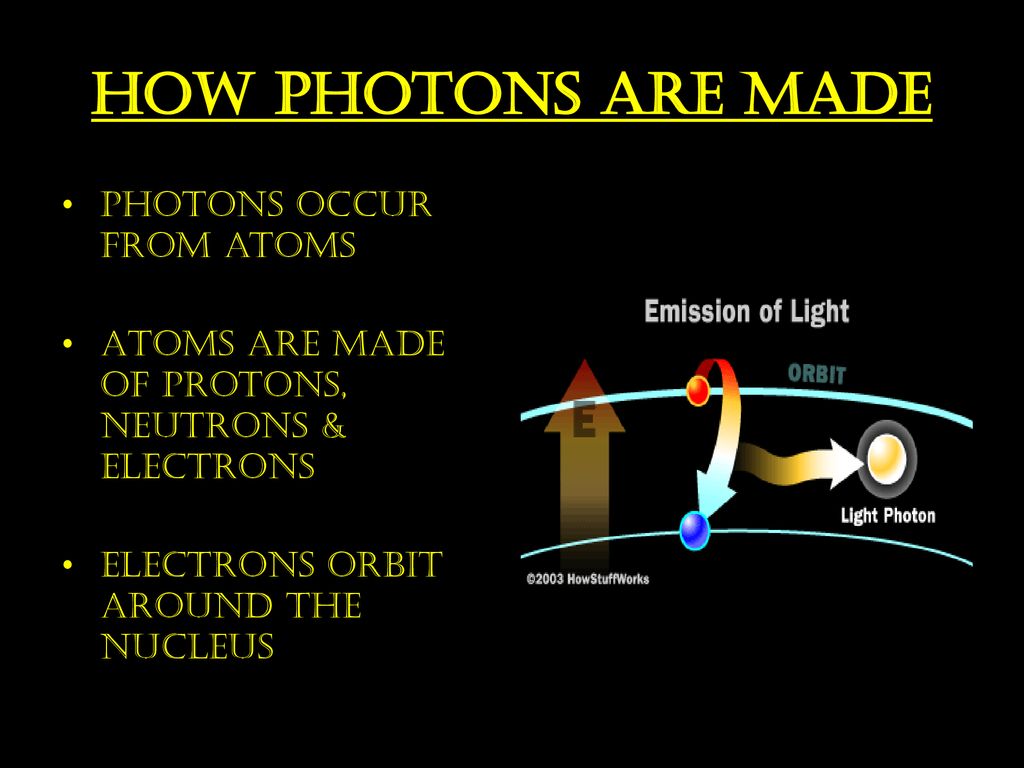
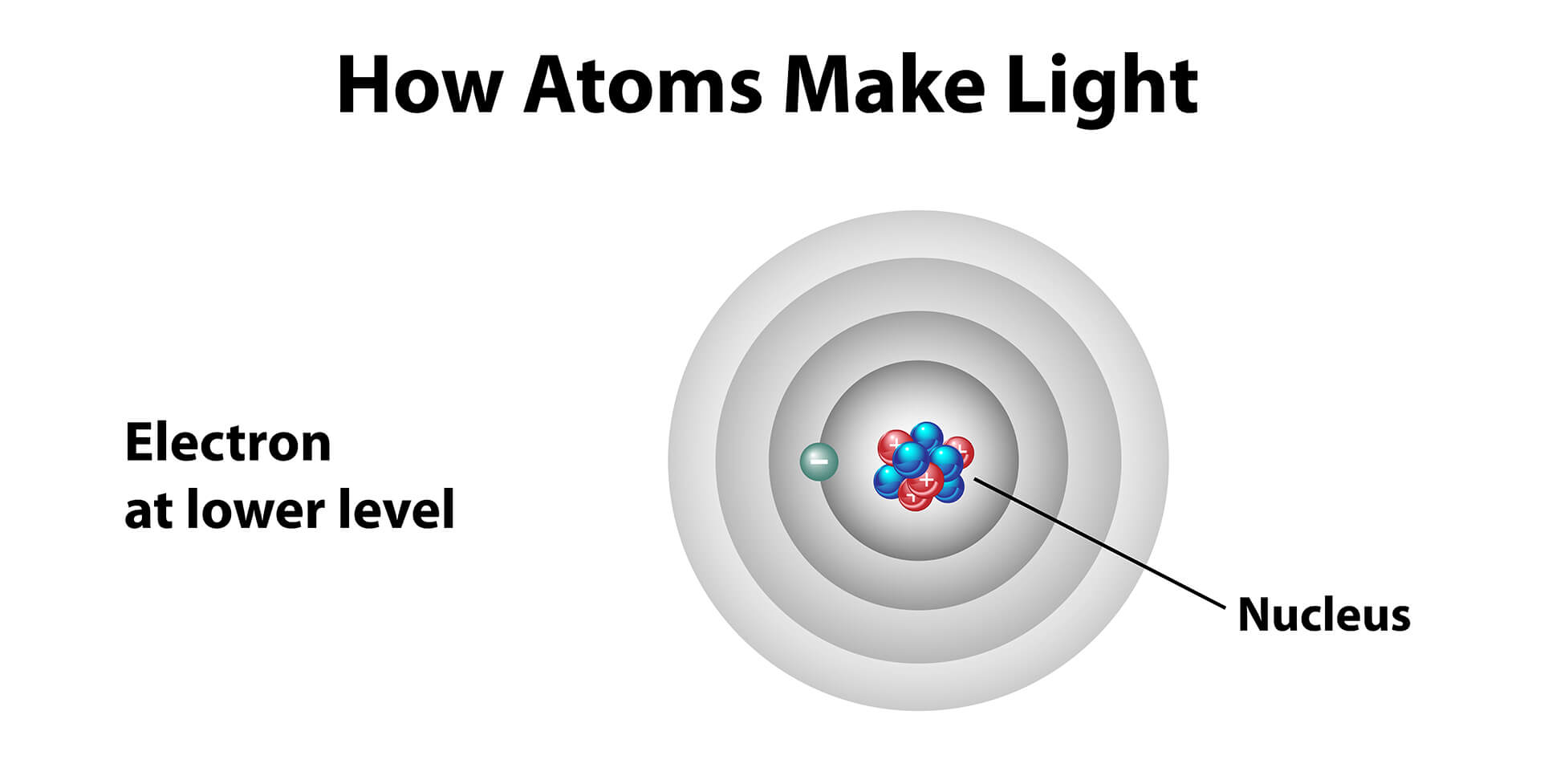
To understand photon emission, we must first venture into the atom itself. An atom, the basic building block of all matter, consists of a nucleus containing protons and neutrons, surrounded by electrons orbiting in specific energy levels or shells.
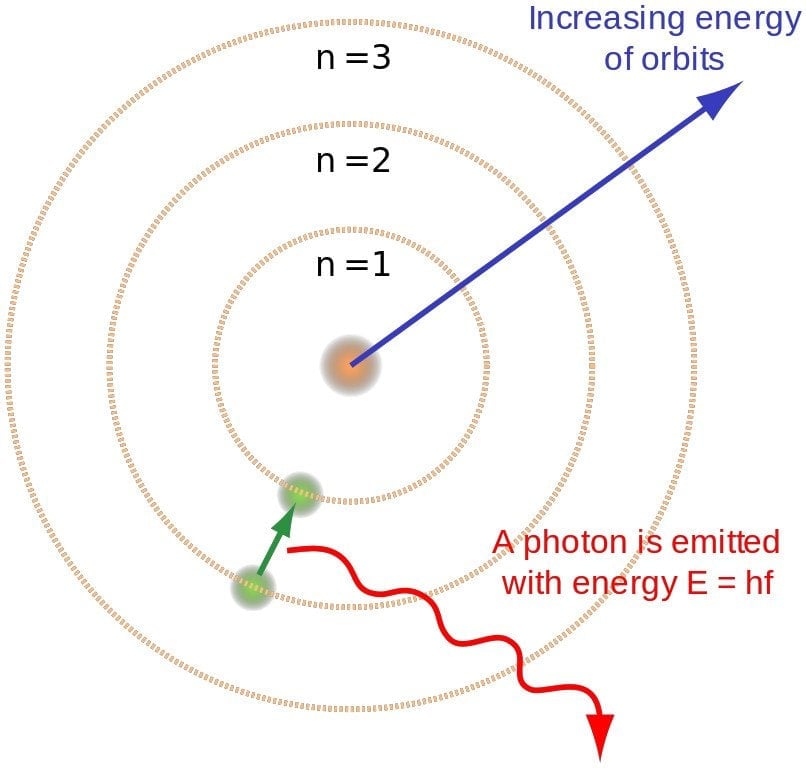
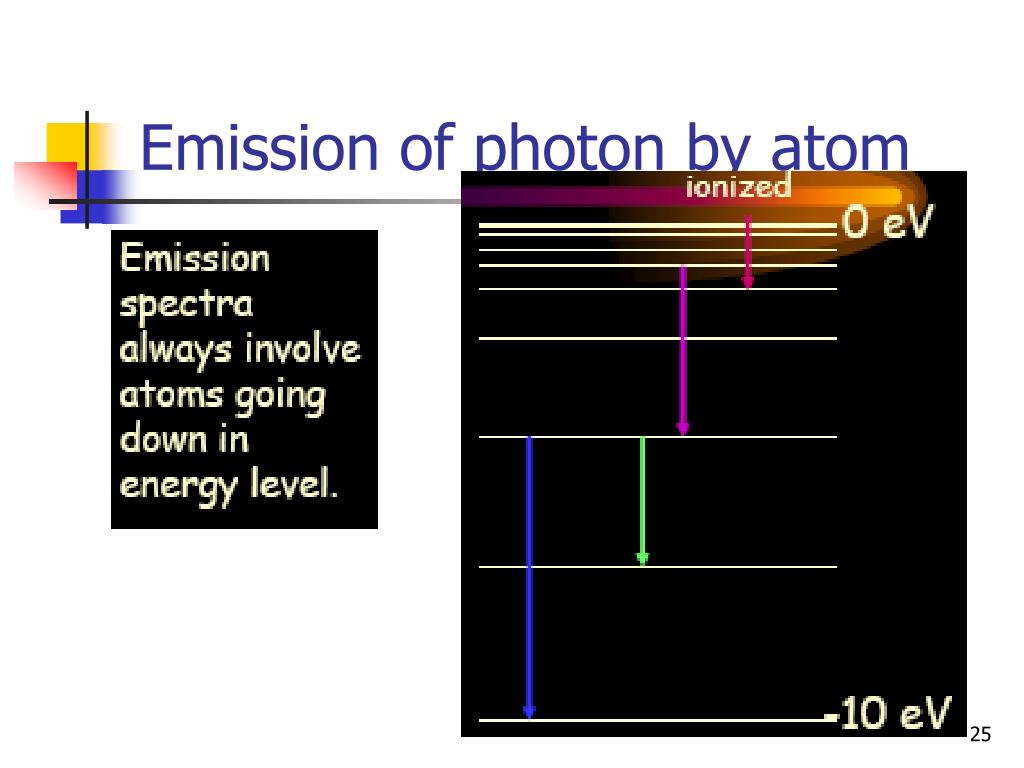
These energy levels are not continuous but are quantized, meaning electrons can only occupy certain discrete energy states. Think of it like a ladder where an electron can only stand on specific rungs, not in between.
Normally, electrons reside in the lowest possible energy level, called the ground state. It's a state of stability, like a perfectly balanced rock.
Excitation: Kicking the Rock
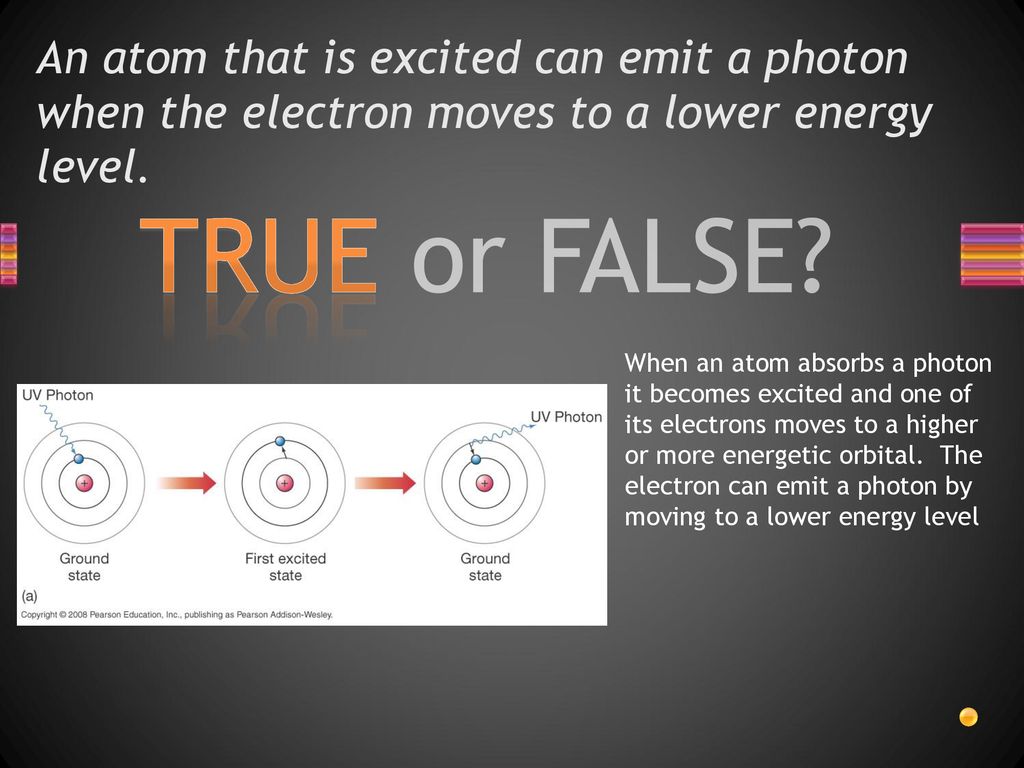
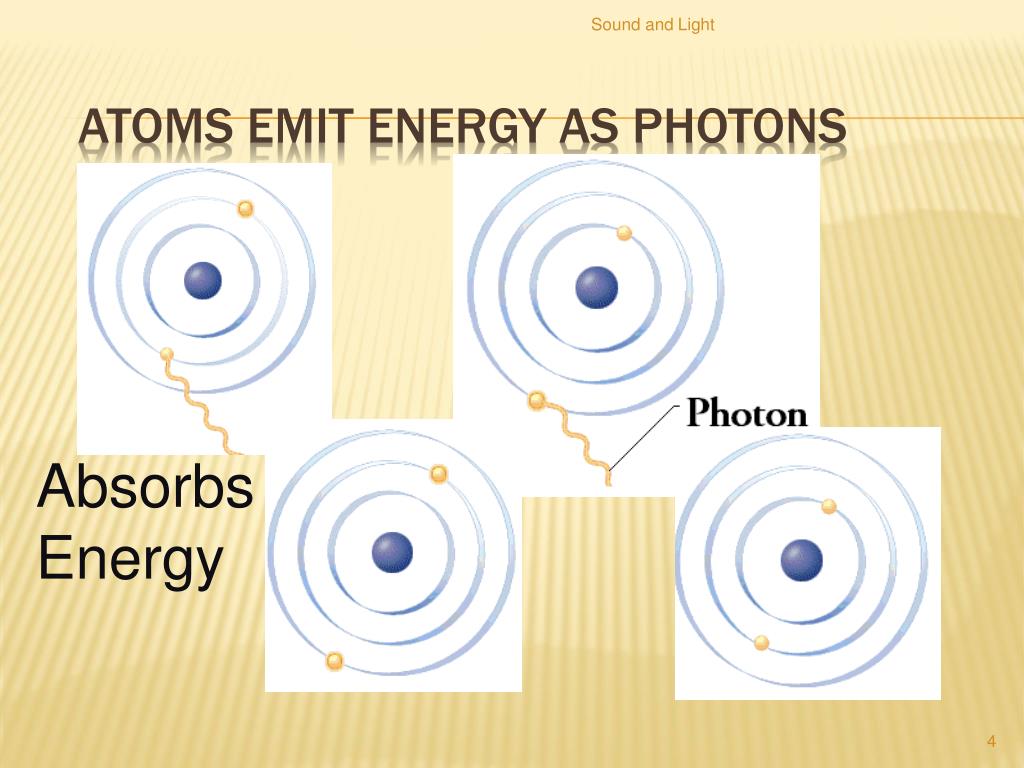
So, how does an electron leave its comfortable ground state? The answer lies in excitation. This occurs when an atom absorbs energy from an external source.
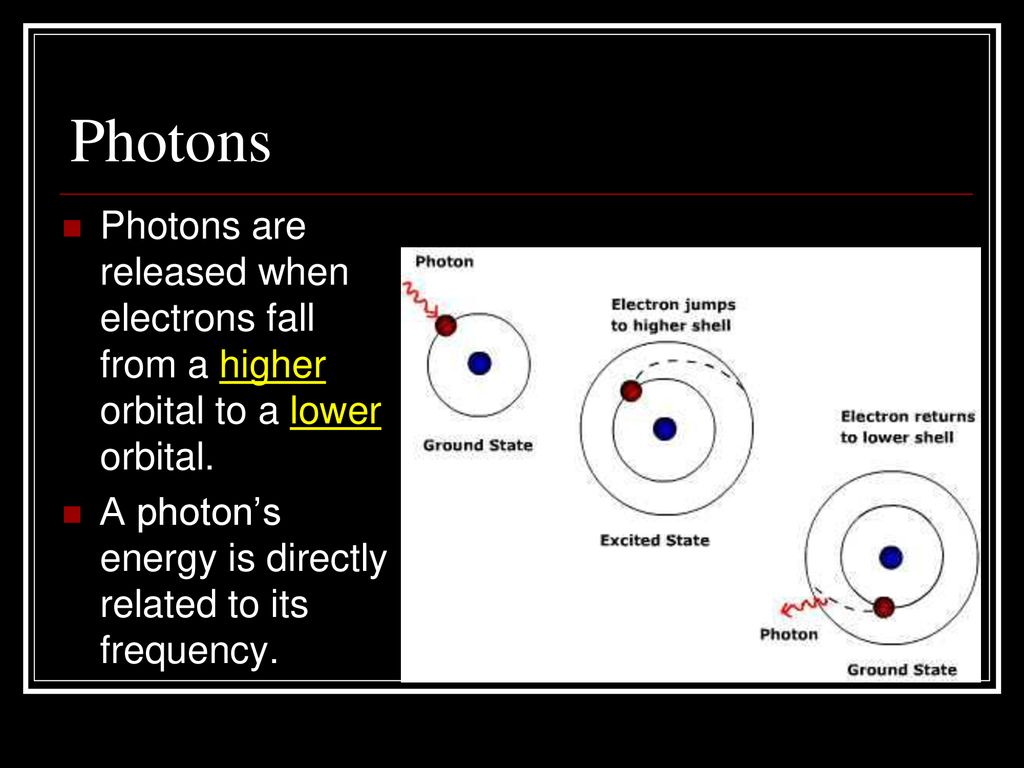
This energy can come in various forms, such as heat, light, or even collisions with other particles. The atom absorbs this energy, causing one of its electrons to jump to a higher energy level, a rung higher up the ladder.
Think of it like giving the rock a kick, sending it tumbling upwards. The atom is now in an excited state, a state of disequilibrium.
The Fall and the Flash: Photon Emission
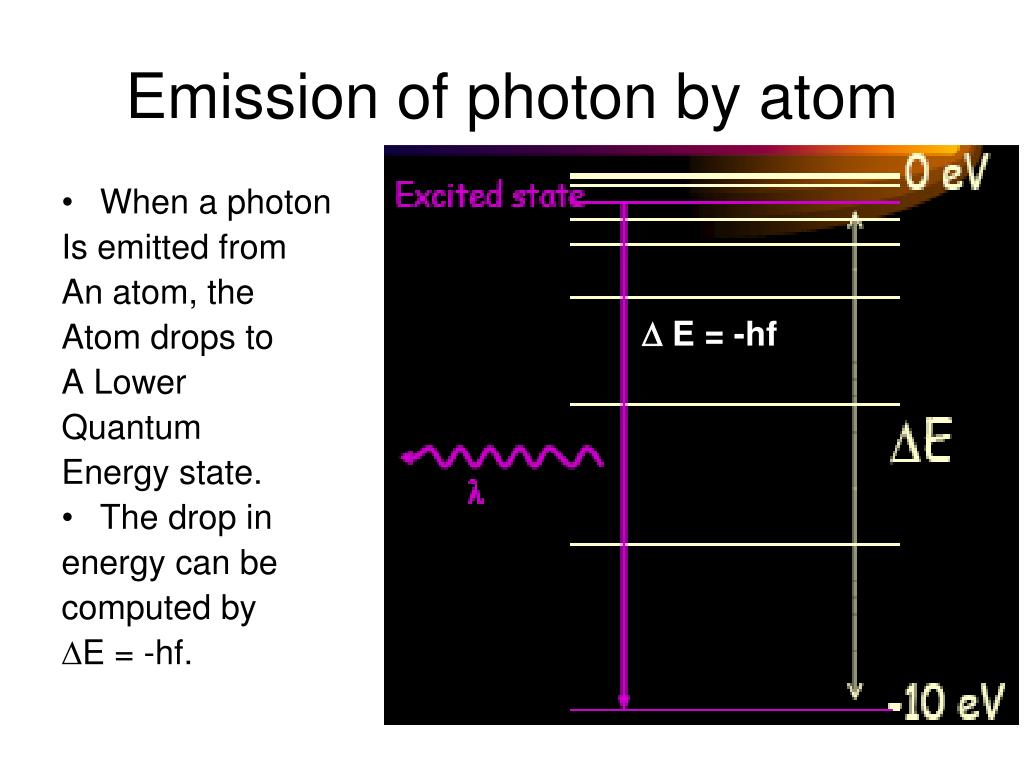
However, an excited state is inherently unstable. The electron, eager to return to its lower energy state, will eventually fall back down. When it does, it releases the energy it absorbed in the form of a photon, a particle of light.
The energy of the emitted photon is precisely equal to the difference in energy between the two energy levels the electron transitioned between. This is a direct consequence of energy conservation, a fundamental principle of physics.
The color of the emitted light is directly related to the energy of the photon: high-energy photons correspond to blue or violet light, while lower-energy photons correspond to red or infrared light. This relationship is governed by the equation E = hv, where E is the energy, h is Planck's constant, and v is the frequency of the light.
Spontaneous vs. Stimulated Emission
Photon emission can occur in two ways: spontaneously or stimulated. Spontaneous emission is, as the name suggests, a random process.
An electron in an excited state will naturally decay back to its ground state after a certain amount of time, emitting a photon in a random direction. This is the process behind the light emitted by light bulbs and most other everyday light sources.
Stimulated emission, on the other hand, is a more controlled process. It occurs when an excited atom is struck by a photon of the exact same energy that it would emit if it were to decay spontaneously.
This incoming photon triggers the excited electron to immediately fall back to its ground state, emitting a photon that is identical to the incoming photon in terms of energy, phase, and direction. This process is the principle behind lasers, devices that produce highly coherent and focused beams of light.
Quantum Mechanics and Probability
It's important to note that the emission of a photon is ultimately governed by the laws of quantum mechanics. This means that we cannot predict exactly when an individual atom will emit a photon, but we can calculate the probability of emission occurring within a given time frame.
The concept of probability is central to quantum mechanics. We deal with probabilities, not certainties, when describing the behavior of atoms and subatomic particles.
This inherent uncertainty is one of the most fascinating and challenging aspects of quantum mechanics, and it has profound implications for our understanding of the nature of reality.
Applications and Significance
The phenomenon of photon emission is not just a theoretical curiosity; it has a wide range of practical applications. From lighting and displays to medical imaging and telecommunications, photon emission plays a crucial role in many technologies that we rely on every day.
Spectroscopy, the study of the interaction of light with matter, relies heavily on the principle of photon emission. By analyzing the spectrum of light emitted by a substance, scientists can determine its composition and physical properties.
This technique is used in a variety of fields, from astronomy to environmental monitoring. For example, astronomers can analyze the light emitted by distant stars to determine their temperature, composition, and velocity. The European Southern Observatory (ESO) uses this method extensively in their studies.
In medicine, fluorescence imaging uses fluorescent molecules that emit light when excited by specific wavelengths. This technique is used to visualize cells and tissues, helping doctors diagnose and treat diseases.
A Fundamental Process
The emission of a photon is a fundamental process that reveals the underlying quantum nature of the universe. It connects the microscopic world of atoms and electrons with the macroscopic world of light and color.
It is a reminder that energy is quantized, that electrons exist in discrete energy levels, and that the emission of light is a consequence of transitions between these levels.
The next time you see a firefly blinking in the night, remember the intricate quantum dance that is taking place within its tiny body, a dance that brings light to the darkness and illuminates the fundamental principles that govern our universe.
..jpg)