What Is Not True About Enzymes

Enzymes, the biological catalysts that speed up chemical reactions in living organisms, are often portrayed in simplified terms. This can lead to widespread misconceptions about their nature and function. Understanding what's not true about enzymes is crucial for accurate scientific literacy and informed decision-making in areas like medicine, biotechnology, and nutrition.
This article aims to debunk some common myths surrounding enzymes. It relies on scientific literature and expert insights to provide a clearer, more nuanced understanding of these essential biomolecules. By addressing these misconceptions, we hope to foster a more accurate and informed view of enzymes.
Enzymes Are Not Always Proteins
A common misconception is that all enzymes are proteins. While the vast majority of enzymes are indeed proteins, there are exceptions to this rule. Ribozymes, which are catalytic RNA molecules, demonstrate that enzymes can also be composed of ribonucleic acid.
The discovery of ribozymes challenged the long-held belief that only proteins could possess catalytic activity. This broadened our understanding of the fundamental principles of biological catalysis. It also opened up new avenues for research in areas like RNA-based therapeutics and synthetic biology.
Enzymes Do Not Get Used Up in Reactions
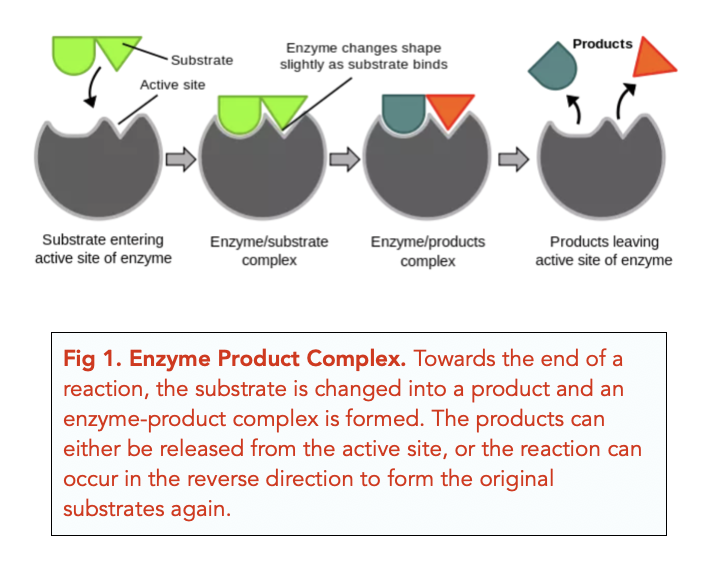
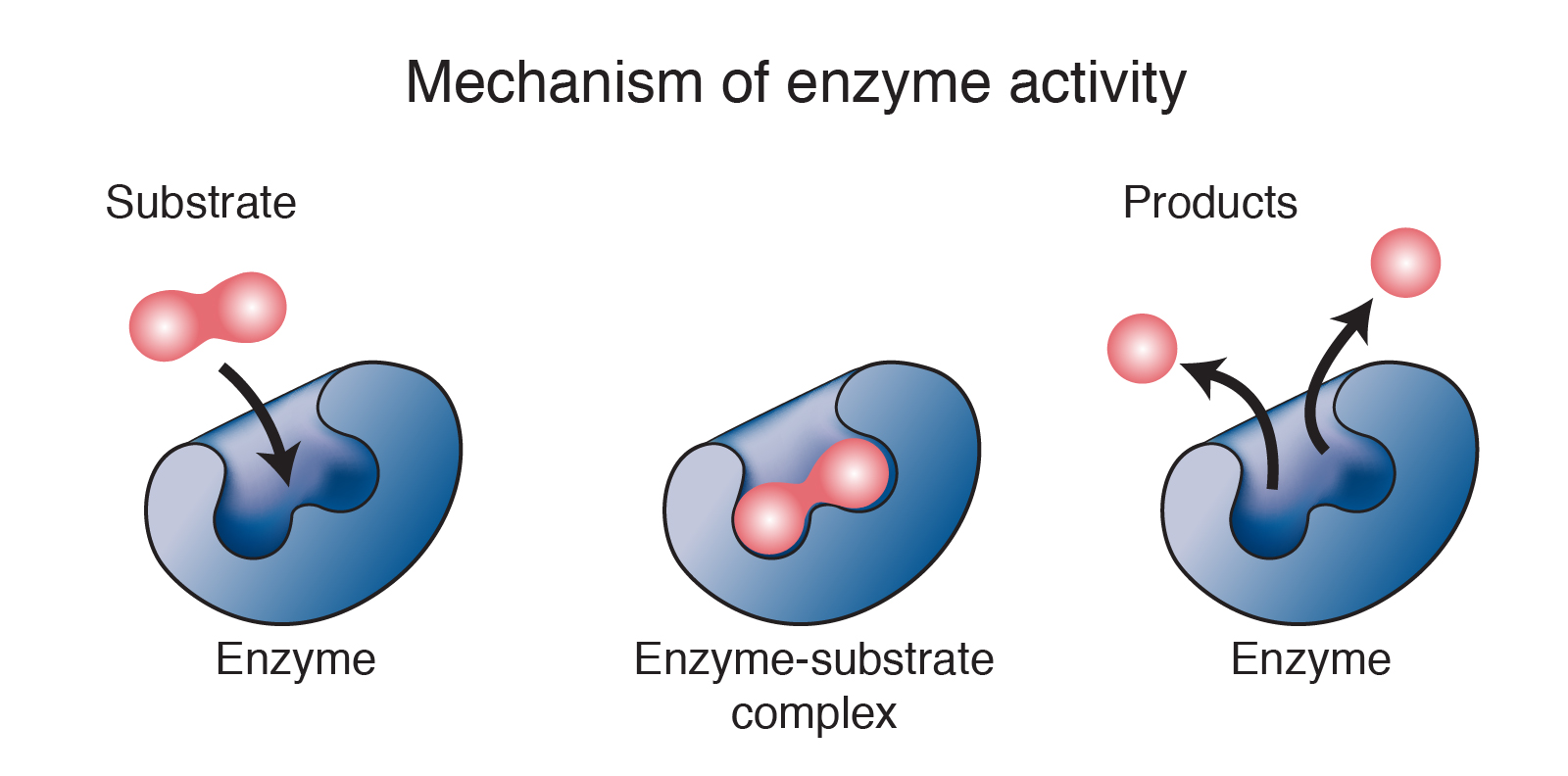
Another widespread misunderstanding is that enzymes are consumed or used up during the reactions they catalyze. This is untrue; enzymes act as catalysts, meaning they speed up reactions without being permanently altered themselves. Once an enzyme has facilitated a reaction, it's free to catalyze another reaction of the same type.
This property is crucial to the efficiency of biological systems. A single enzyme molecule can catalyze thousands or even millions of reactions over its lifetime. This recycling ability makes enzymes incredibly efficient catalysts.
Enzymes Do Not Work in Isolation
Enzymes are often depicted as working in isolation, but in reality, they frequently operate within complex metabolic pathways. These pathways consist of a series of interconnected enzymatic reactions, where the product of one reaction becomes the substrate for the next. This coordinated activity allows cells to carry out complex biochemical processes.
Understanding the interconnectedness of enzymatic reactions is essential for comprehending cellular metabolism. Targeting a single enzyme in a metabolic pathway can have far-reaching effects on other reactions within the pathway. This concept is relevant to drug development and the understanding of metabolic diseases.
Enzymes Are Not Infinitely Specific
While enzymes are known for their specificity, they are not always absolutely specific for a single substrate. Some enzymes can catalyze reactions with a range of structurally similar substrates. This phenomenon is known as substrate promiscuity.
Substrate promiscuity can be advantageous in some situations, allowing enzymes to adapt to different conditions or to catalyze novel reactions. However, it can also lead to unwanted side reactions if the enzyme interacts with unintended substrates. This is an active area of research. It impacts fields like enzyme engineering and drug metabolism.
Enzymes Do Not Only Speed Up Reactions
It is true that enzymes speed up the rates of biochemical reactions, but this statement needs further elaboration. They accelerate reactions by lowering the activation energy required for a reaction to occur. They do not change the equilibrium of the reaction itself.
Enzymes only affect the rate at which equilibrium is reached, not the final ratio of reactants to products. This distinction is crucial for understanding the thermodynamics of enzyme-catalyzed reactions. It ensures that reactions proceed towards their thermodynamically favored outcome.
Enzyme Activity Is Not Constant
Enzyme activity is not a fixed property; it can be regulated by various factors, including temperature, pH, and the presence of inhibitors or activators. Changes in these factors can significantly affect the rate at which an enzyme catalyzes a reaction.
The regulation of enzyme activity is essential for maintaining cellular homeostasis. Cells can respond to changing conditions by modulating the activity of key enzymes. This allows them to adapt their metabolism to meet the demands of their environment.
The Significance of Addressing These Misconceptions
Addressing these misconceptions is vital for promoting a deeper understanding of biology and biochemistry. It's also important for advancing scientific research and innovation. By clarifying the nature of enzymes, we can avoid misunderstandings in related fields such as medicine, nutrition, and biotechnology.
For example, a more nuanced understanding of enzyme specificity and regulation can inform the development of more effective drugs. Correcting misconceptions about enzyme activity can help individuals make informed choices about dietary supplements and their impact on health.
Future Directions
Further research is needed to explore the complexities of enzyme structure, function, and regulation. Advanced techniques, such as X-ray crystallography and molecular dynamics simulations, can provide valuable insights into the inner workings of enzymes. It can also improve our ability to design and engineer enzymes for specific applications.
Education and outreach efforts are crucial for disseminating accurate information about enzymes to the general public. By promoting a deeper understanding of these essential biomolecules, we can empower individuals to make informed decisions and to appreciate the wonder of life at the molecular level.
In conclusion, while enzymes are often presented in a simplified manner, it's important to recognize the nuances of their function. They aren't always proteins, aren't consumed in reactions, don't work in isolation, aren't infinitely specific, don't only speed up reactions, and their activity isn't constant. A more accurate understanding of enzymes is crucial for various fields, impacting everything from medicine to biotechnology.