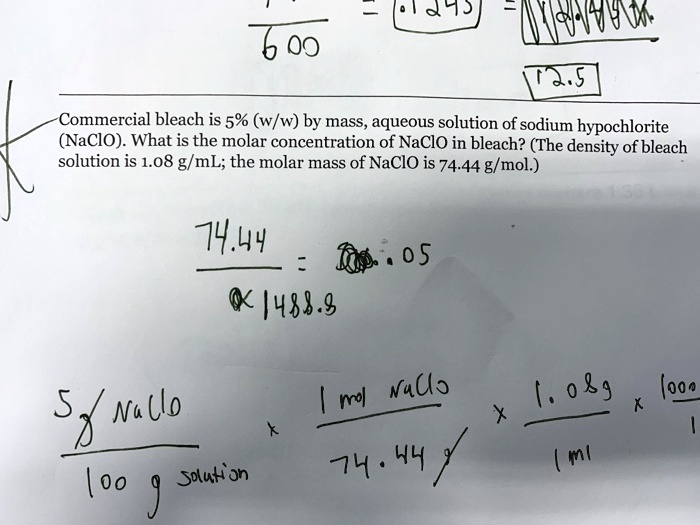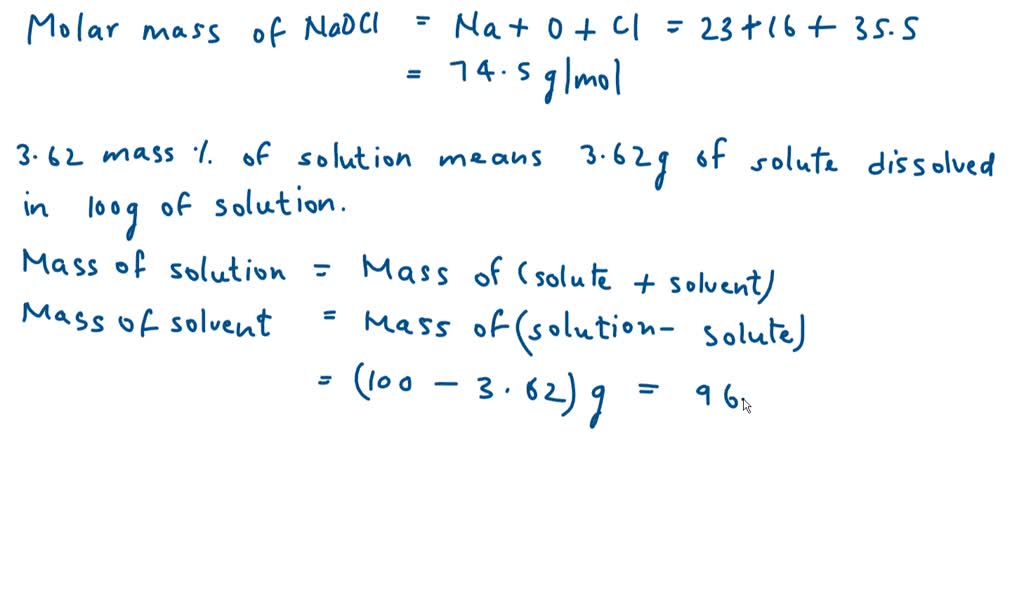What Is The Molarity Of A Bleach Solution Containing 9.5

A potentially dangerous lack of clarity surrounds the concentration of common household bleach. Determining the molarity of a bleach solution is crucial for safe and effective use, particularly in cleaning, sanitation, and laboratory settings.
The widely variable concentration of active ingredient, sodium hypochlorite (NaOCl), in commercially available bleach products makes a 'one-size-fits-all' answer impossible. This article clarifies how to calculate the molarity of a bleach solution containing 9.5% NaOCl.
Understanding Molarity and Bleach Composition
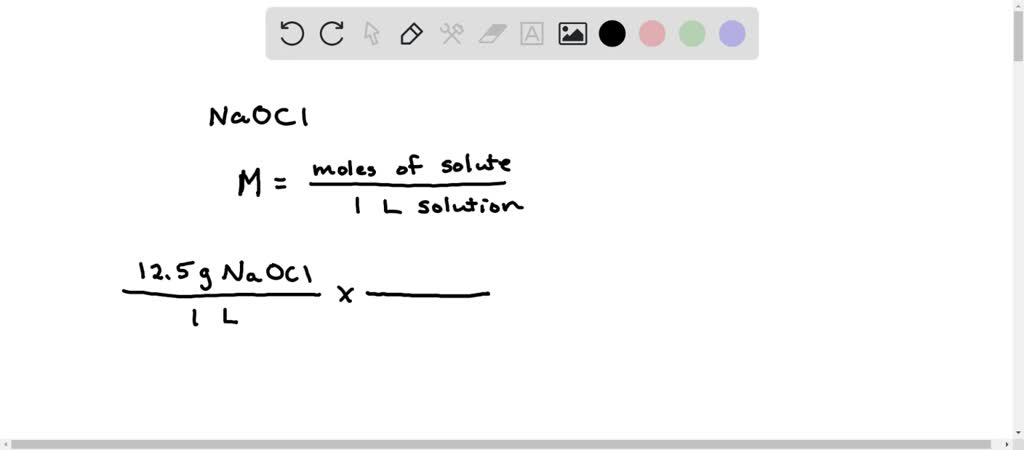
Molarity (M) represents the number of moles of solute per liter of solution. For bleach, the solute is sodium hypochlorite (NaOCl).
Most household bleach solutions contain varying percentages of NaOCl, typically ranging from 3% to 10%. The higher the percentage, the stronger the bleach and the higher the molarity.
Calculating Molarity: The Step-by-Step Process
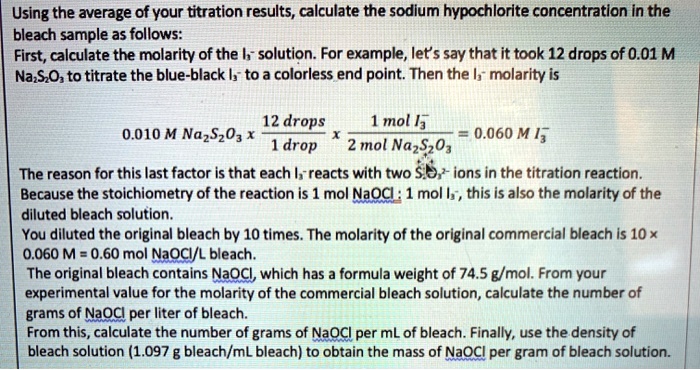
For a bleach solution containing 9.5% NaOCl, we need to convert this percentage into molarity. This requires a series of conversions.
First, assume we have 100 grams of the bleach solution. 9.5% NaOCl means 9.5 grams of NaOCl are present in those 100 grams of solution.
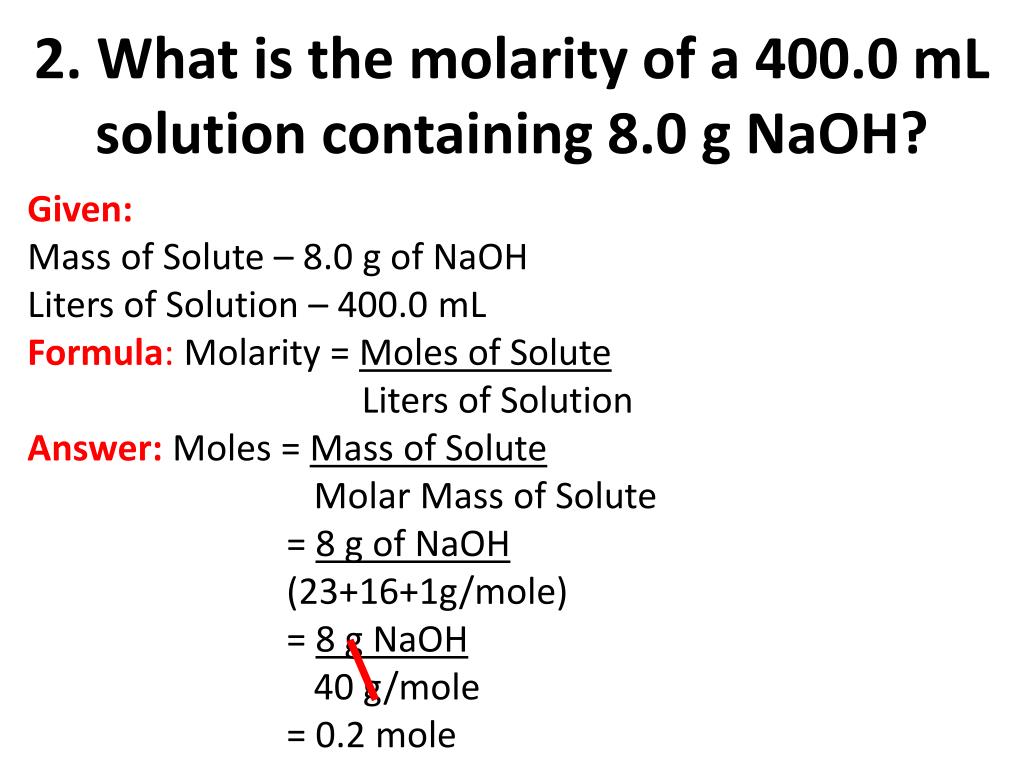
Next, convert grams of NaOCl to moles. The molar mass of NaOCl is approximately 74.44 g/mol.
Therefore, 9.5 grams of NaOCl is equal to 9.5 g / 74.44 g/mol = 0.128 moles of NaOCl.
Now, we need to convert the mass of the solution (100 grams) to volume (liters). We'll assume the density of the bleach solution is approximately 1.1 g/mL. This can vary slightly depending on the specific formulation.
Using the density, 100 grams of solution is equivalent to 100 g / 1.1 g/mL = 90.9 mL. Convert mL to liters: 90.9 mL = 0.0909 L.
Finally, calculate the molarity: Molarity = moles of solute / liters of solution. Molarity = 0.128 moles / 0.0909 L = 1.41 M.
Implications and Safety Considerations
A 9.5% bleach solution has a molarity of approximately 1.41 M. This is a significant concentration and requires careful handling.
Always dilute bleach appropriately for specific applications. Undiluted bleach can be corrosive and harmful.
Wear appropriate personal protective equipment (PPE), such as gloves and eye protection, when handling bleach.
Never mix bleach with ammonia or other cleaning agents. This can create toxic and potentially deadly gases.
Verification and Ongoing Research
The calculated molarity of 1.41 M is an approximation based on assumed density and ideal conditions. Actual molarity may vary slightly depending on the specific brand and formulation of the bleach.
Consulting the Material Safety Data Sheet (MSDS) for the specific bleach product you are using is always recommended.
Further research is underway to develop more precise and accessible methods for determining the concentration of household bleach. This includes exploring the use of titration kits and spectroscopic techniques for consumer use.
For critical applications or situations where precise concentration is essential, consider using commercially prepared sodium hypochlorite solutions with a certified concentration, or consulting with a qualified chemist.