Which List Includes Only Physical Properties
In classrooms and boardrooms, from elementary school science labs to advanced materials engineering facilities, the fundamental understanding of physical properties reigns supreme. But when presented with lists containing a mix of characteristics, discerning which one *solely* encapsulates these inherent traits can become surprisingly complex. The consequences of misidentification ripple through various fields, affecting quality control, research accuracy, and even consumer understanding of products.
The seemingly simple question – "Which list includes only physical properties?" – unveils a deeper need for clarity and precision in scientific literacy. This article dissects the core definition of physical properties, explores common points of confusion, and examines how this knowledge impacts industries and everyday life. We will delve into real-world examples and expert opinions to provide a comprehensive understanding of this critical concept.
Defining Physical Properties: The Core Concepts
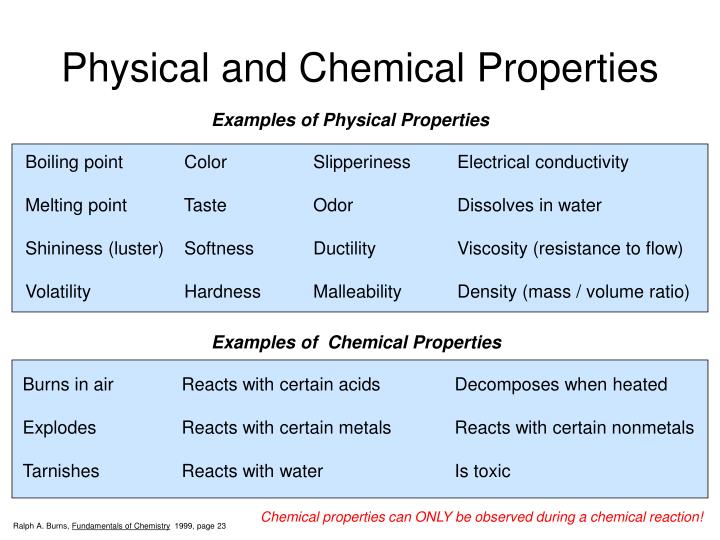
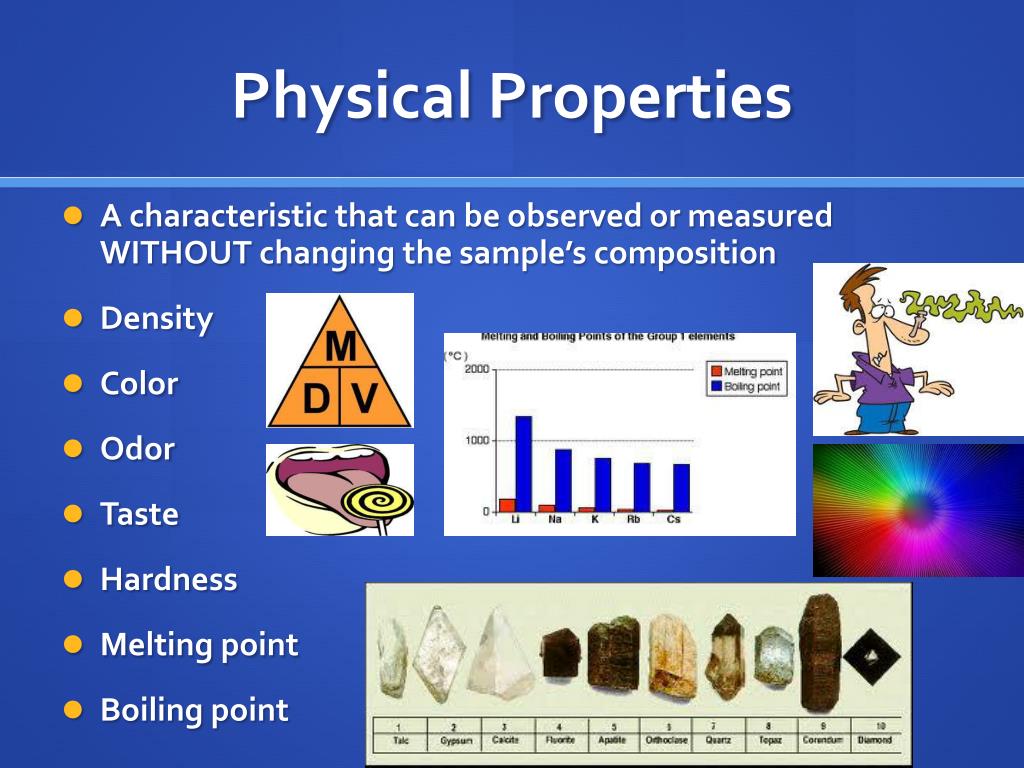
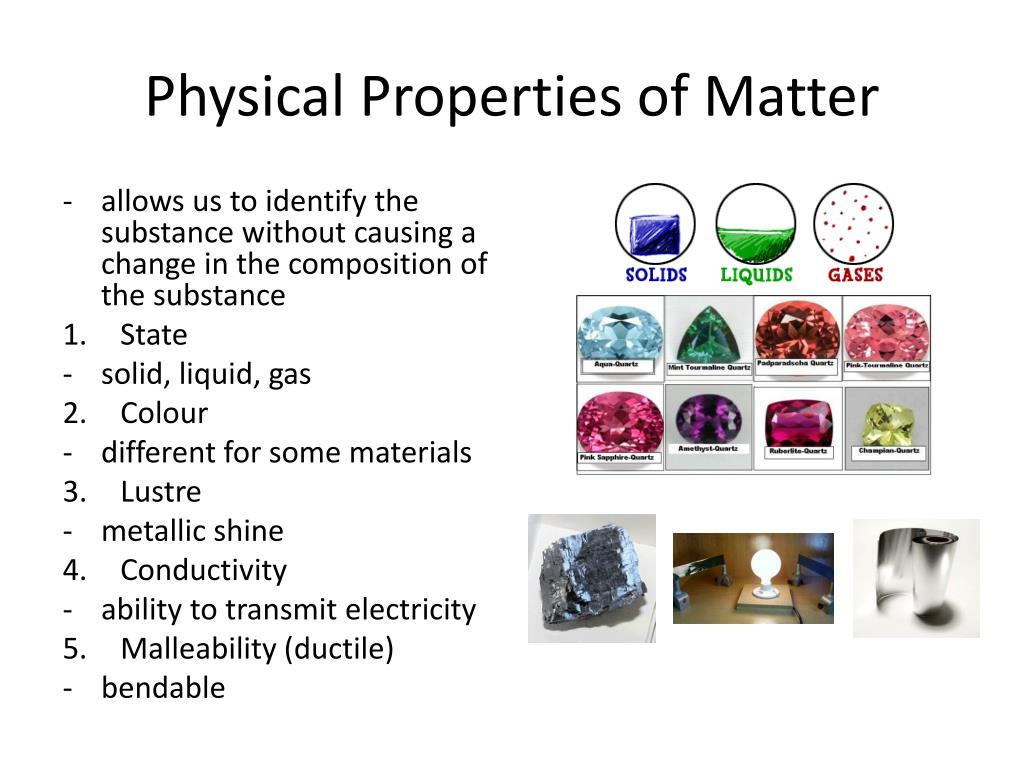
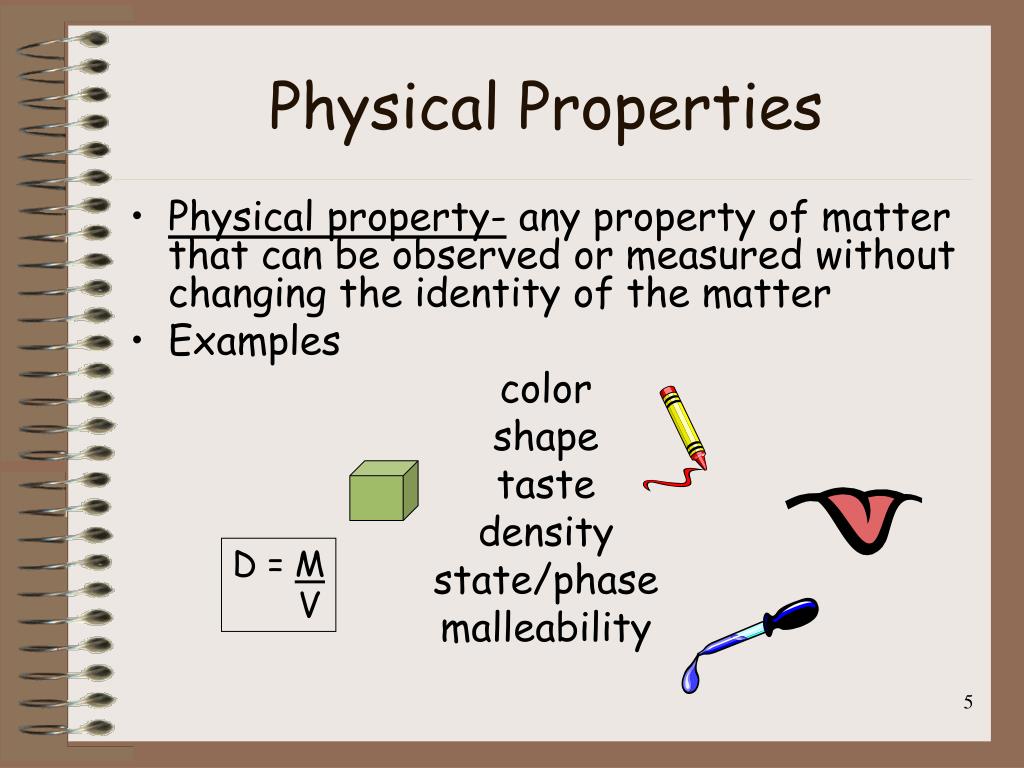
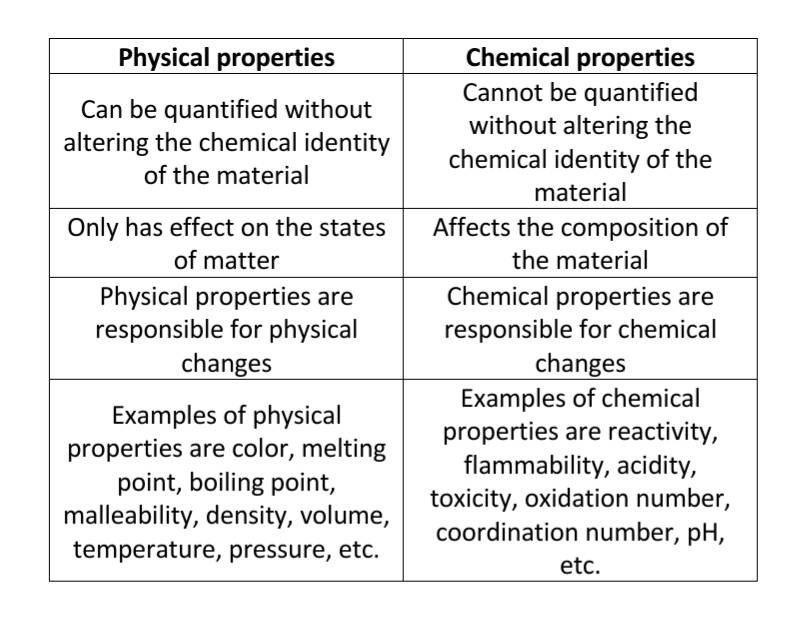
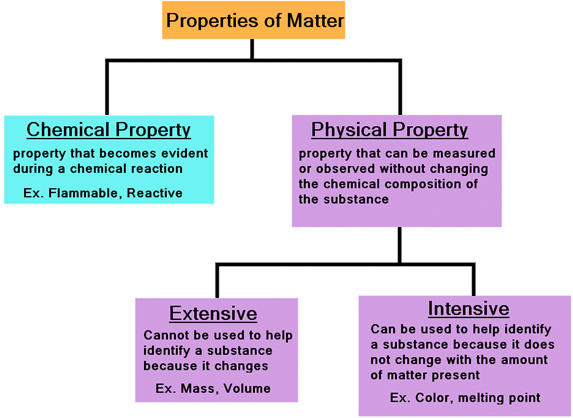
Physical properties are characteristics of a substance that can be observed or measured without changing the substance's chemical composition. These properties describe the state of the matter itself, not its potential to react or transform into something new. The absence of chemical change is the crucial differentiator.
Consider the difference between flammability (a *chemical property*) and density (a *physical property*). Flammability describes a substance's ability to burn and transform into other substances, requiring a chemical reaction. Density, on the other hand, is the mass per unit volume and can be measured without altering the substance's chemical identity.
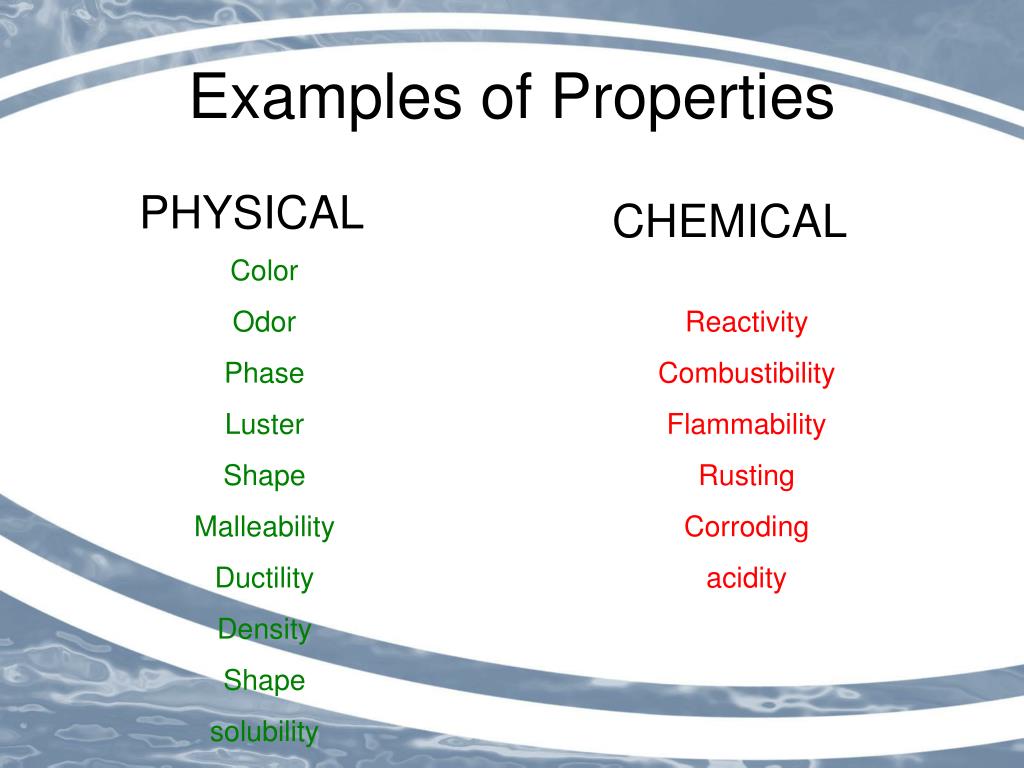
Common Examples of Physical Properties
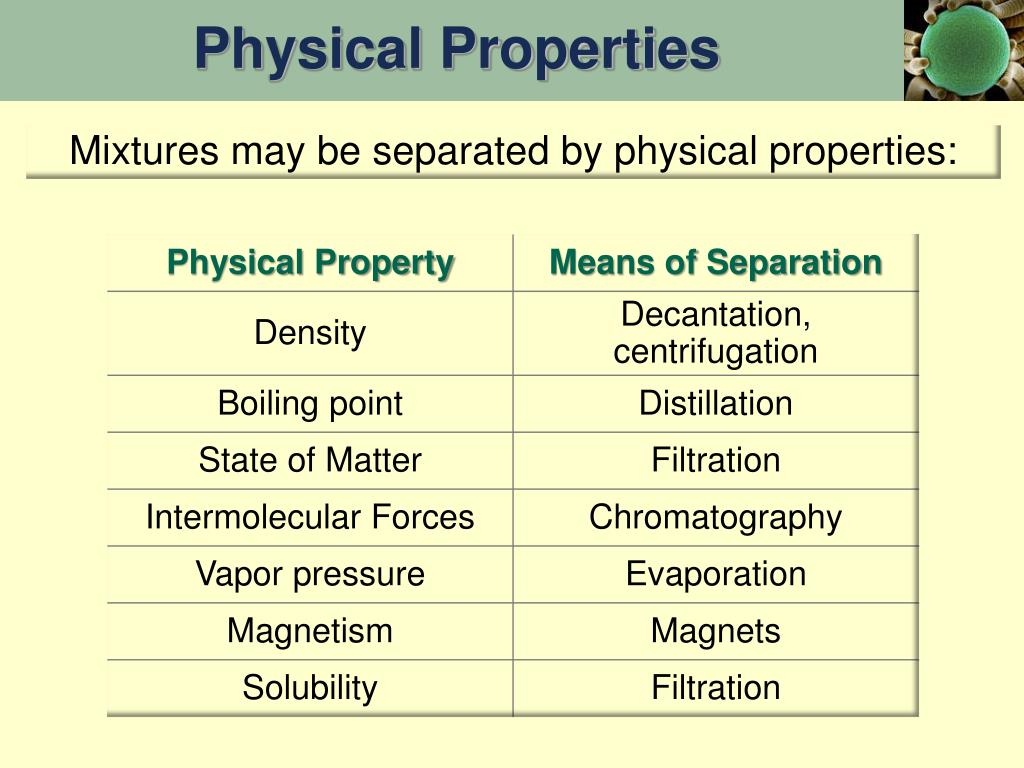
Many physical properties are readily observable. These include color, odor, mass, volume, and density.
Other examples require instrumentation to measure accurately. Examples include melting point, boiling point, conductivity (electrical and thermal), hardness, and solubility.
The National Institute of Standards and Technology (NIST) provides comprehensive databases and standards for measuring and reporting physical properties. NIST is a key resource for ensuring accuracy and consistency across different laboratories and industries.
The Confusion Factor: Chemical vs. Physical
Distinguishing between physical and chemical properties is where errors often occur. The key is to focus on whether the observation or measurement causes a change in the substance's chemical identity.
For example, reactivity is often mistaken for a physical property. Reactivity describes how readily a substance undergoes a chemical reaction, which inherently changes its composition.
Another source of confusion lies in properties that can be both *physical* and *chemical* depending on the context. For instance, acidity can be considered a *physical property* if measured by its pH, but it becomes a *chemical property* when describing its ability to react with a base.
Real-World Applications and Implications
The correct identification of physical properties is paramount in many industries. Material selection in engineering, for example, relies heavily on accurate knowledge of properties like tensile strength (the resistance to breaking under tension) and elasticity (the ability to return to its original shape after deformation).
In the food industry, understanding physical properties like viscosity (resistance to flow) and texture is crucial for product development and quality control. These properties directly affect consumer experience and product shelf life.
Pharmaceutical companies rely on precise measurements of physical properties such as solubility and melting point to ensure drug efficacy and stability. A slight deviation can alter the drug's absorption rate and therapeutic effect.
Expert Perspectives on Common Misconceptions
"One of the biggest challenges we face in teaching introductory chemistry is overcoming the misconception that any observable trait is automatically a physical property," says Dr. Emily Carter, a chemistry professor at the California Institute of Technology (Caltech). She emphasizes the importance of asking: "Does observing this property change the substance itself?"
According to Dr. Michael Chen, a materials scientist at MIT, "The line between physical and chemical properties can sometimes blur, especially when dealing with complex materials." He recommends focusing on the fundamental definitions and always considering the context of the measurement.
"Accurate characterization of physical properties is the foundation of reliable scientific research,"states Dr. Sarah Lee, a research scientist at the National Renewable Energy Laboratory (NREL). She emphasizes the importance of using standardized methods and calibrated instruments.
Looking Ahead: Enhancing Scientific Literacy
Improving scientific literacy regarding physical properties requires a multi-faceted approach. Educational institutions must prioritize clear and concise definitions, coupled with hands-on experiments.
Promoting critical thinking skills is essential for students to differentiate between observation and chemical change. Emphasizing real-world applications and case studies can further solidify understanding.
Ultimately, a deeper understanding of physical properties empowers individuals to make informed decisions, analyze data critically, and contribute meaningfully to scientific advancements. This understanding, built on accurate knowledge and careful distinction, will continue to drive innovation across diverse fields for years to come.