Which Of The Following Is An Intensive Property
:max_bytes(150000):strip_icc()/intensive-vs-extensive-properties-604133-v3-5b55fb394cedfd0037117796.png)
The seemingly simple question, "Which of the following is an intensive property?" often sparks confusion and debate in scientific and engineering circles. The implications of misunderstanding this fundamental concept ripple through various fields, affecting research accuracy, industrial process optimization, and even the design of everyday technologies. Misidentification can lead to flawed experiments, inefficient manufacturing processes, and ultimately, unreliable products.
At its core, the distinction between intensive and extensive properties is crucial for characterizing materials and systems. The ability to correctly identify intensive properties, those that do not depend on the amount of substance present, is essential for scaling up processes, predicting material behavior under varying conditions, and ensuring consistency in experimental results. This article delves into the nature of intensive properties, contrasting them with extensive properties, examining real-world examples, and highlighting their significance across various scientific disciplines.
Understanding Intensive and Extensive Properties
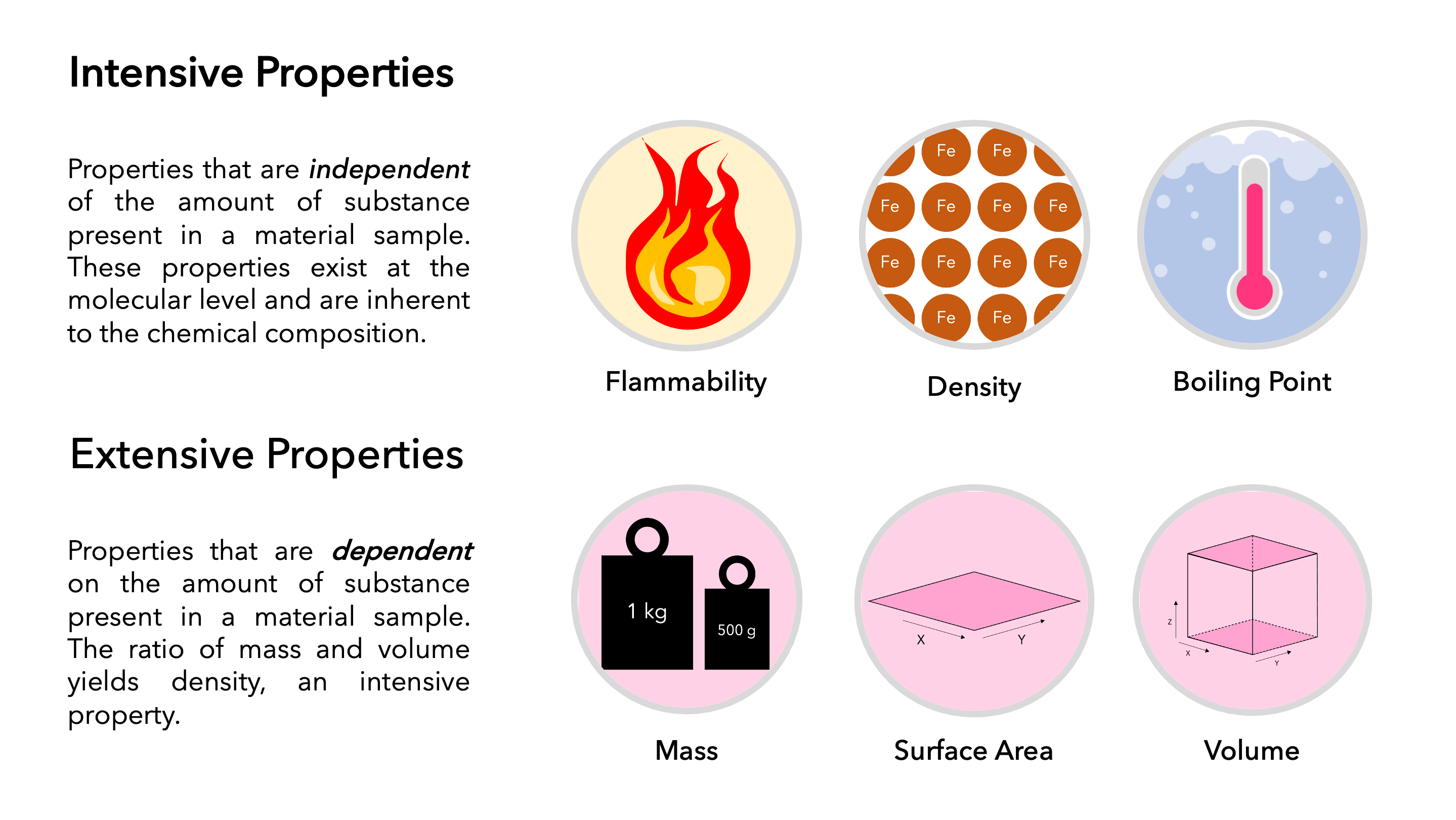
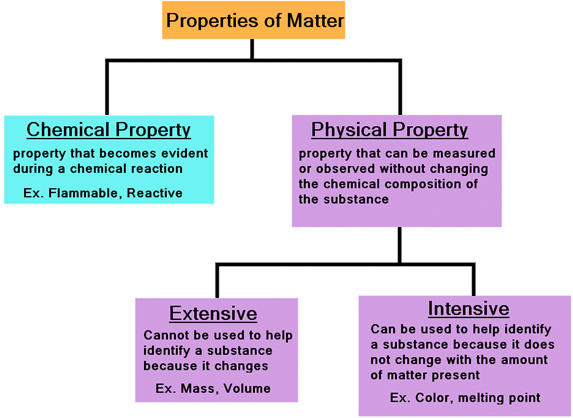
Intensive properties are those that do not change when the amount of substance changes. Think of properties like temperature, pressure, density, and melting point. These values remain constant regardless of how much material you have.
In contrast, extensive properties are directly proportional to the amount of matter. Mass, volume, and energy are classic examples of extensive properties. Doubling the amount of substance will double its mass, volume, and energy.
Key Differences and Examples
The fundamental difference lies in the dependence on the system's size or extent. An intensive property is a bulk property, meaning it's a local physical property of a system that does not depend on the system size or the amount of material in the system. An extensive property will change if the amount of material in the system changes.
Consider a pot of boiling water. The temperature of the water, approximately 100°C (212°F) at sea level, remains the same whether you have a small cup or a large pot of boiling water. Temperature is an intensive property.
However, the amount of heat required to boil the water differs significantly based on the quantity. The larger pot of water requires more energy to reach boiling point, demonstrating that heat (energy) is an extensive property.
Density is another illustrative example of an intensive property. Density is defined as mass per unit volume (ρ = m/V). Even if you have a large quantity of gold or a small nugget, the density of gold (approximately 19.3 g/cm³) remains constant. Volume and mass themselves are extensive properties, but their ratio creates an intensive property.
Real-World Applications and Significance
The distinction between intensive and extensive properties is vital in many fields. In chemistry, intensive properties like boiling point and freezing point are used to identify and characterize substances. These properties are critical in separating mixtures and purifying compounds through distillation or crystallization.
In engineering, understanding intensive properties is crucial for designing and optimizing industrial processes. For instance, knowing the specific heat capacity (an intensive property) of a material allows engineers to calculate the amount of energy required to heat it to a certain temperature, a critical consideration in process design.
Material science also heavily relies on these concepts. Material properties like tensile strength and hardness, though often influenced by factors like processing and microstructure, are inherently intensive properties. They describe the material's inherent resistance to deformation or failure, regardless of the sample's size.
Impact on Research and Development
In research, accurately identifying and measuring intensive properties ensures reproducibility and comparability of experimental results. If researchers are studying the properties of a new material, they must report intensive properties that are independent of the sample size. This allows other researchers to replicate the experiment and confirm the findings, even with different quantities of the material.
Failing to distinguish between intensive and extensive properties can lead to significant errors in data interpretation and analysis. For example, if a researcher incorrectly attributes a change in heat capacity (extensive) to a change in the material's inherent properties (intensive), they may draw incorrect conclusions about the material's behavior.
The International Union of Pure and Applied Chemistry (IUPAC) provides standardized definitions and guidelines for physical quantities and units, emphasizing the importance of clear distinction between intensive and extensive properties for scientific communication and data reporting. Adhering to these standards promotes consistency and accuracy in research.
Common Misconceptions and Challenges
One common misconception is that all physical properties are either strictly intensive or strictly extensive. In reality, some properties can exhibit characteristics of both, depending on the specific system and conditions.
Surface tension, for example, is often considered an intensive property because it's a characteristic of the liquid itself. However, the total surface energy (an extensive property) depends on the surface area, which is related to the amount of liquid.
Another challenge arises when dealing with non-homogeneous systems. In these cases, intensive properties may vary from one region to another within the system. For example, the density of a mixture of oil and water will differ depending on whether you measure it in the oil phase or the water phase.
The Future of Property Characterization
Advancements in analytical techniques and computational modeling are enabling more precise and comprehensive characterization of material properties. Sophisticated instruments and simulations allow researchers to probe materials at increasingly smaller scales, revealing subtle variations in intensive properties that were previously undetectable.
The rise of data-driven materials science is further emphasizing the importance of accurate property characterization. Machine learning algorithms can be trained on large datasets of material properties to predict the behavior of new materials and optimize existing ones. The reliability of these predictions hinges on the quality and accuracy of the input data, including the correct identification and measurement of intensive and extensive properties.
Ultimately, a strong understanding of intensive properties is crucial for driving innovation in various fields. By correctly identifying and utilizing these properties, scientists and engineers can develop new materials, optimize industrial processes, and create technologies that improve our lives. Continued education and awareness in this area will ensure that future research and development efforts are based on a solid foundation of fundamental principles.