Which Of The Following Is True Regarding The Ph Scale

The pH scale, a cornerstone of chemistry and environmental science, is frequently misunderstood, leading to inaccurate interpretations of its implications. From assessing water quality to understanding bodily functions, its accurate application is vital. Misconceptions surrounding the scale can have far-reaching consequences, affecting decisions in agriculture, medicine, and industry.
This article aims to clarify the fundamental truths about the pH scale, addressing common misunderstandings and highlighting its practical significance. We will delve into the scale's definition, its range, and its logarithmic nature, ensuring readers gain a solid understanding of this critical scientific tool. By consulting scientific literature and expert opinions, we will explore the key facts about the pH scale.
Understanding the Basics of pH
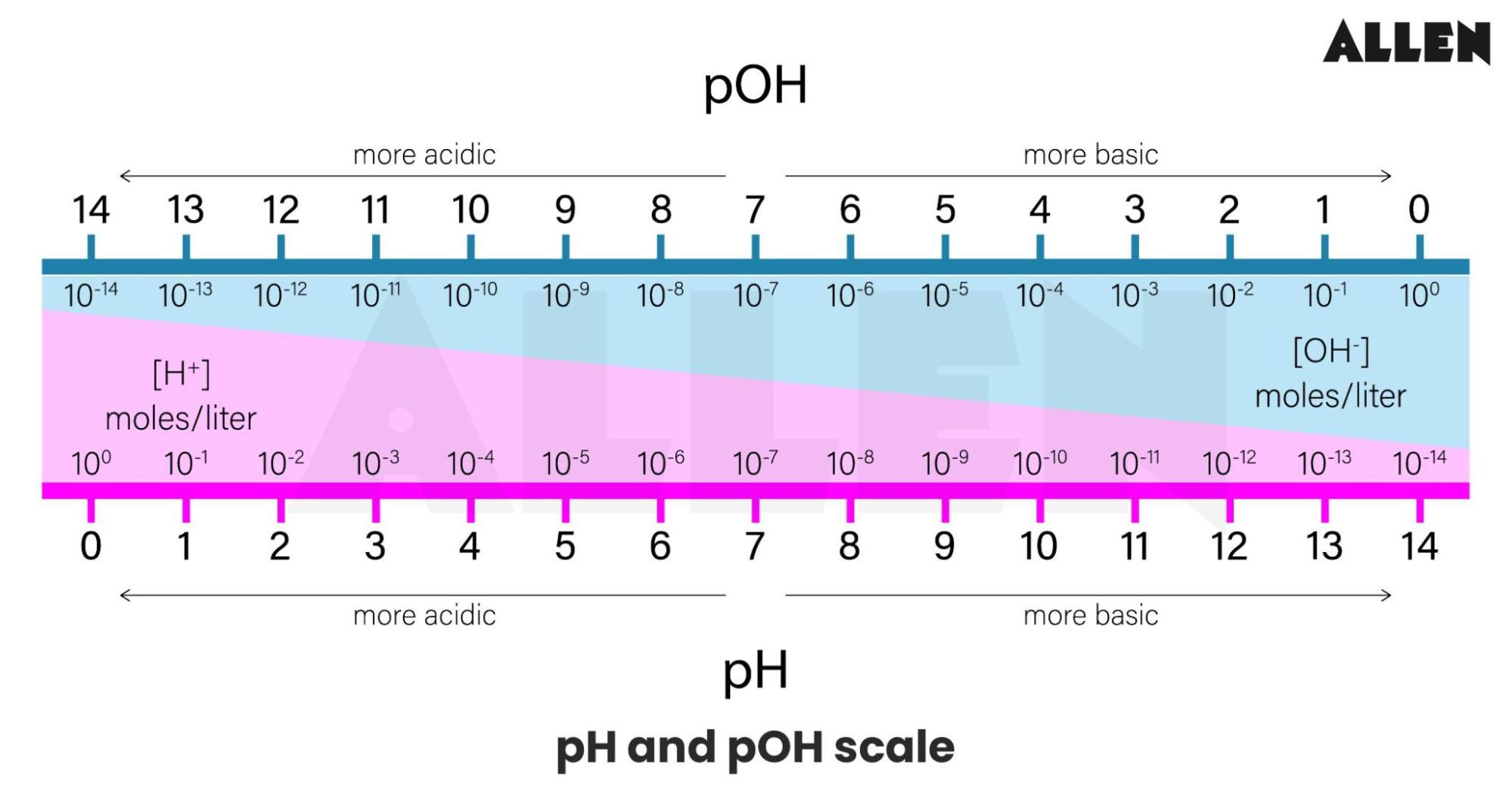
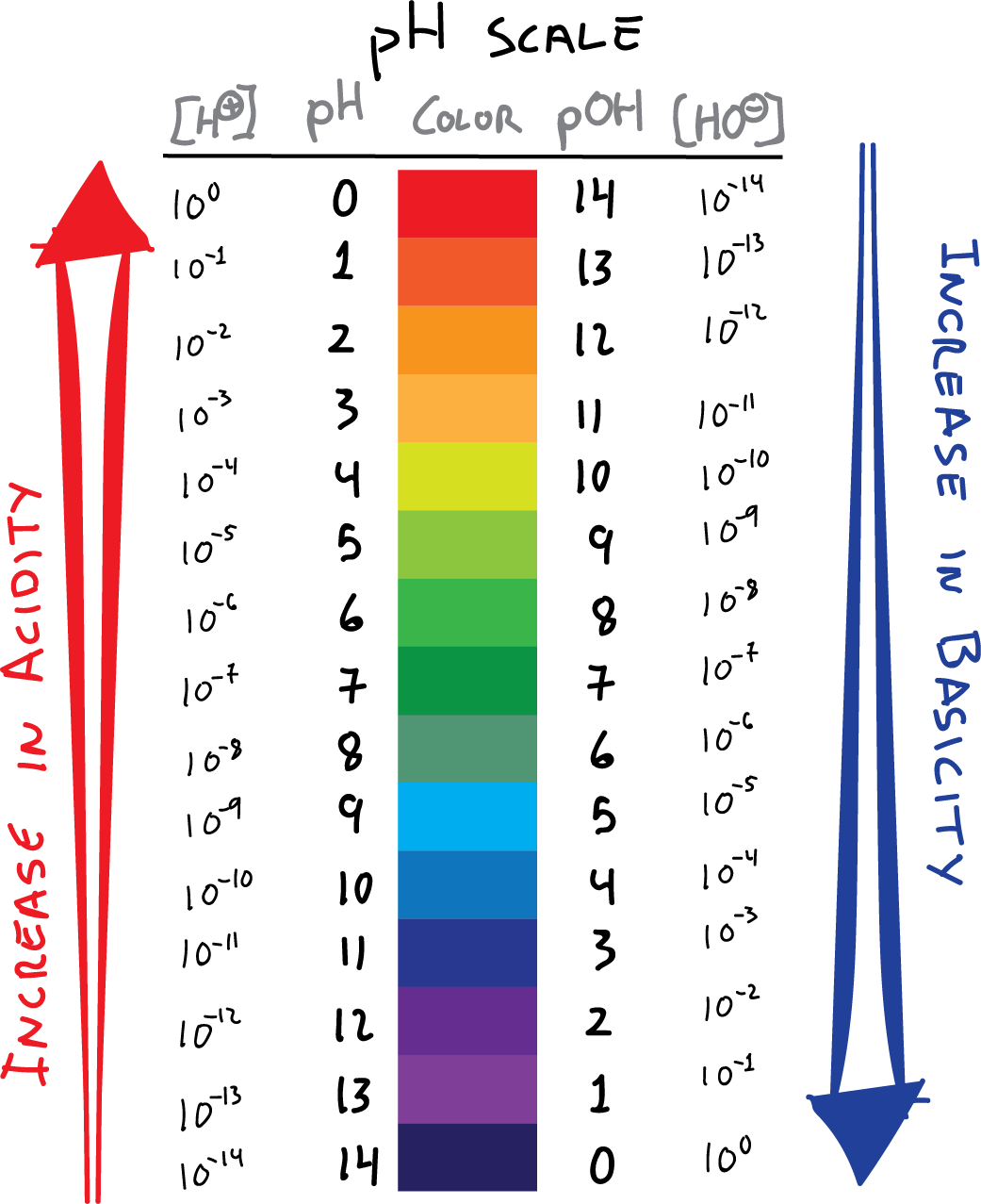
The pH scale is a measure of the hydrogen ion concentration ([H+]) in a solution. Specifically, it represents the negative logarithm (base 10) of the hydrogen ion activity. It's a quantitative measure of the acidity or basicity of aqueous or other liquid solutions.
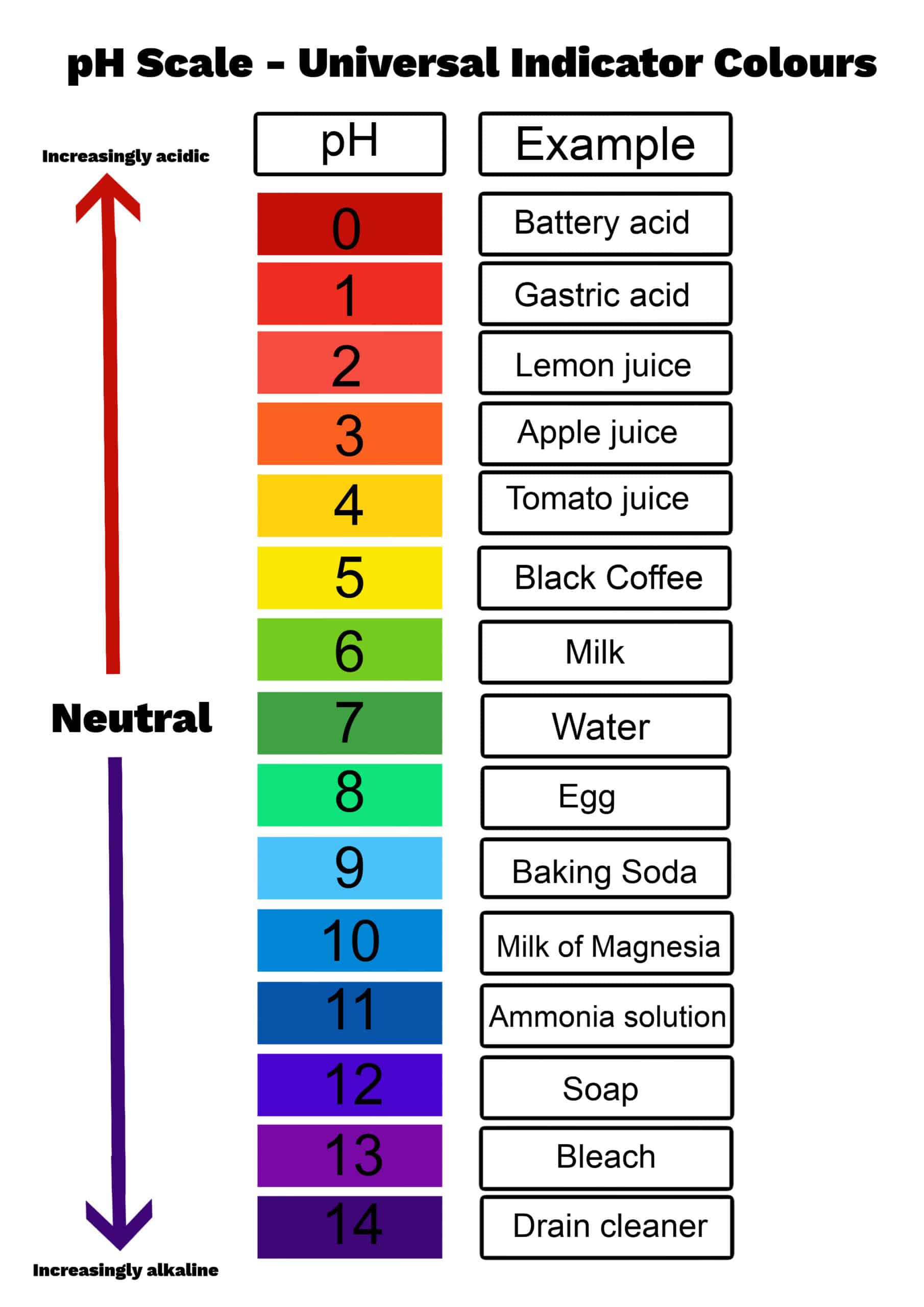
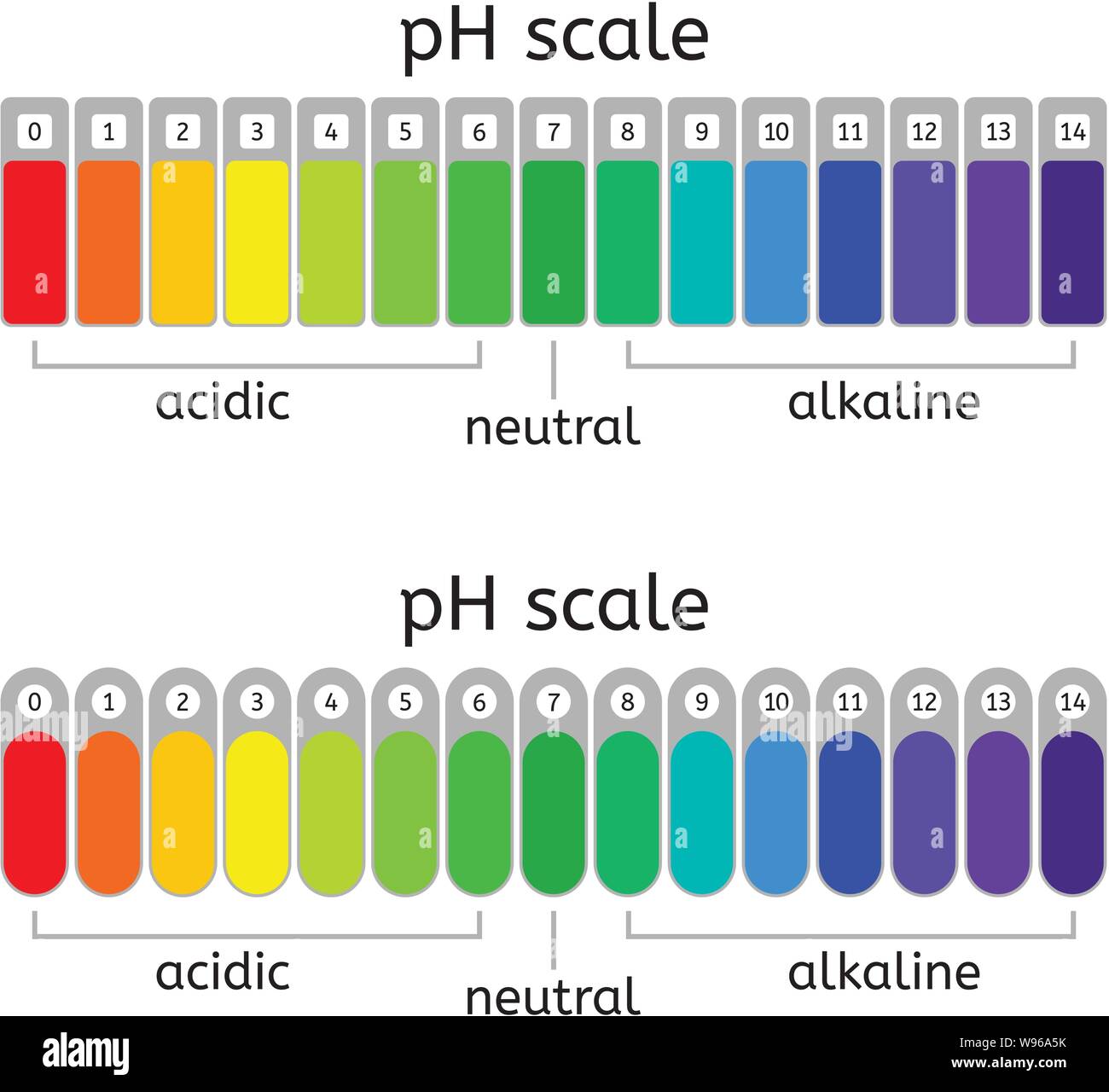
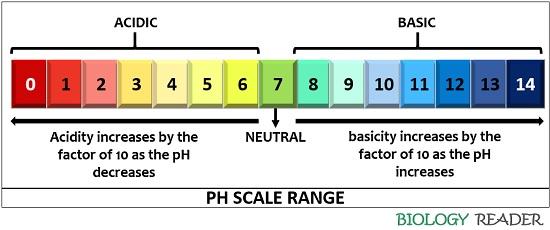
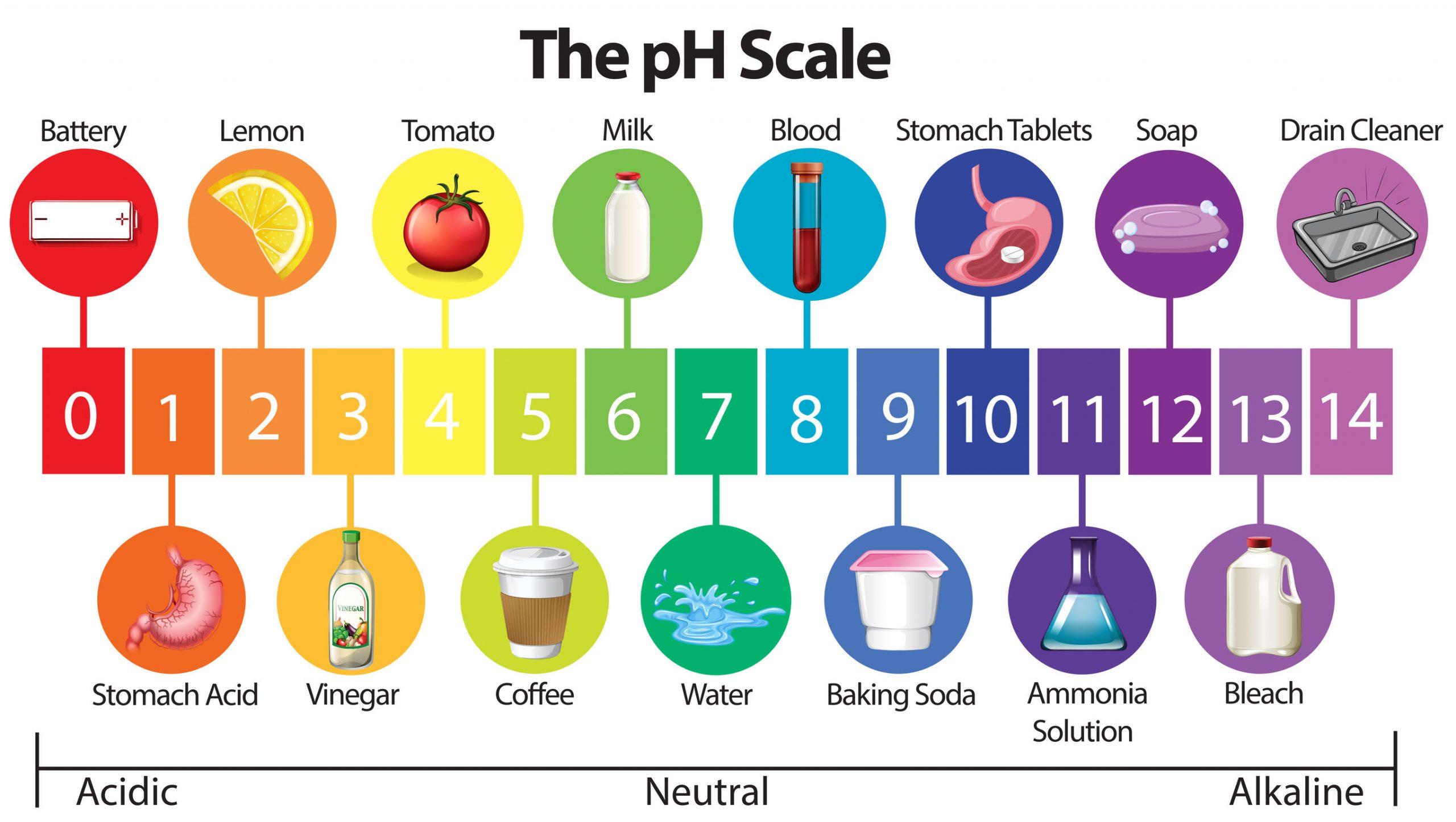
pH values typically range from 0 to 14, although values slightly outside this range are possible in highly concentrated solutions. A pH of 7 is considered neutral, indicating equal concentrations of hydrogen and hydroxide ions.
Values below 7 indicate acidity, with lower numbers representing stronger acids. Values above 7 signify alkalinity (or basicity), with higher numbers indicating stronger bases.
Key Facts and Misconceptions
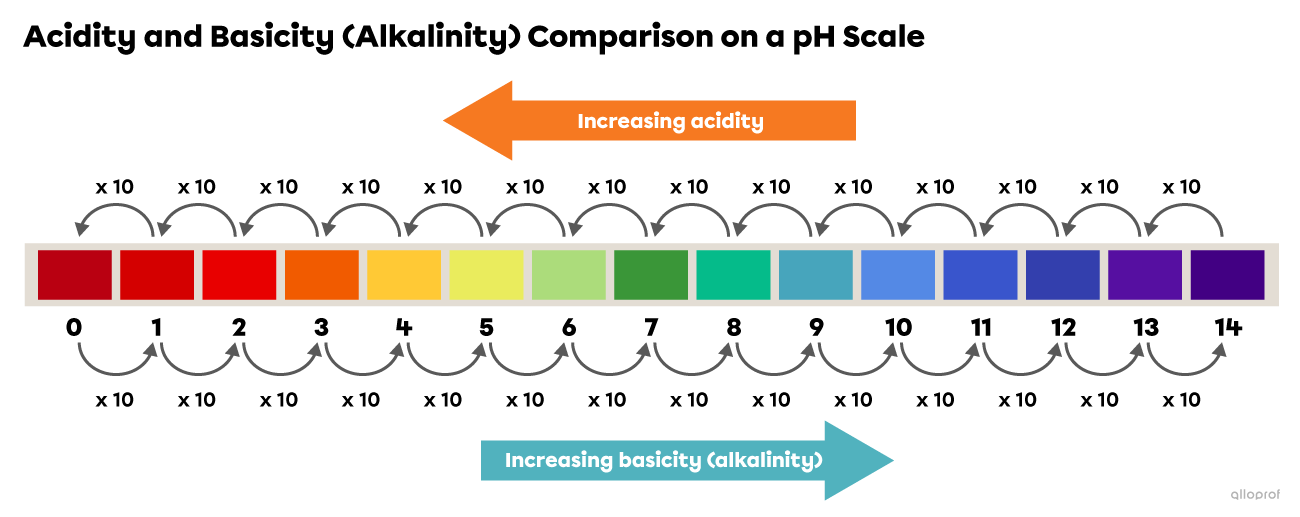
A common misconception is that the pH scale is linear. In reality, the scale is logarithmic, meaning each whole pH value below 7 is ten times more acidic than the next higher value.
For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4, and one hundred times more acidic than a solution with a pH of 5. This logarithmic nature is crucial for understanding the dramatic changes in acidity or alkalinity reflected by even small pH shifts.
Another crucial aspect is that pH is temperature-dependent. The pH of pure water is exactly 7 at 25°C (77°F). However, as temperature increases, the dissociation of water increases, leading to a slight decrease in pH, while remaining neutral.
The Scale and Solution Properties
The pH of a solution directly affects its chemical properties. Highly acidic solutions can be corrosive, reacting with metals and other materials.
Highly alkaline solutions can also be corrosive, often feeling slippery to the touch. Understanding the pH of a solution is critical in determining its suitability for various applications.
For instance, the pH of soil affects nutrient availability for plants, while the pH of blood is tightly regulated to ensure proper bodily function. Maintaining a balanced pH is essential for many biological and industrial processes.
Methods for Measuring pH
There are several methods for measuring pH, each with its own advantages and limitations. Litmus paper and other pH indicator papers provide a quick, approximate pH measurement.
These papers contain dyes that change color depending on the pH of the solution. However, their accuracy is limited.
Electronic pH meters, which use a glass electrode to measure the hydrogen ion concentration, offer much greater precision. These meters require calibration with known buffer solutions to ensure accurate readings.
The Importance of Accurate pH Measurement
Accurate pH measurement is crucial in a variety of fields. In agriculture, soil pH affects nutrient availability and plant growth, so farmers regularly monitor soil pH to optimize crop yields.
In medicine, blood pH is a critical indicator of health, with deviations from the normal range (7.35-7.45) indicating serious medical conditions. In industry, pH control is essential in many chemical processes, ensuring product quality and safety.
For example, in wastewater treatment, pH adjustment is necessary to remove pollutants and protect aquatic life. The US Environmental Protection Agency (EPA) sets pH limits for discharged water to protect the environment.
"pH is a critical parameter for assessing water quality," notes a recent EPA report.
Looking Ahead
Future research may focus on developing more accurate and reliable pH sensors, particularly for use in challenging environments. Nanomaterials and other advanced technologies could lead to sensors that are smaller, more robust, and less susceptible to interference.
Furthermore, there is growing interest in using pH sensors for real-time monitoring of environmental conditions and industrial processes. This would allow for faster detection of problems and more effective corrective action.
In conclusion, the pH scale is a fundamental concept with wide-ranging applications. A thorough understanding of its logarithmic nature, temperature dependence, and implications for solution properties is essential for accurate interpretation and effective use. By dispelling common misconceptions and highlighting the importance of accurate measurement, we can ensure that the pH scale continues to serve as a valuable tool in science, industry, and beyond.


+is+another+way+of+representing+the+hydrogen+ion+(H%2B)+concentration+of+a+solution..jpg)
![Which Of The Following Is True Regarding The Ph Scale PH scale The pH scale is used to measure the [H+] and [OH-] in solution](https://slideplayer.com/slide/14956017/91/images/1/pH+scale+The+pH+scale+is+used+to+measure+the+[H%2B]+and+[OH-]+in+solution.+[H%2B]+[OH-].jpg)