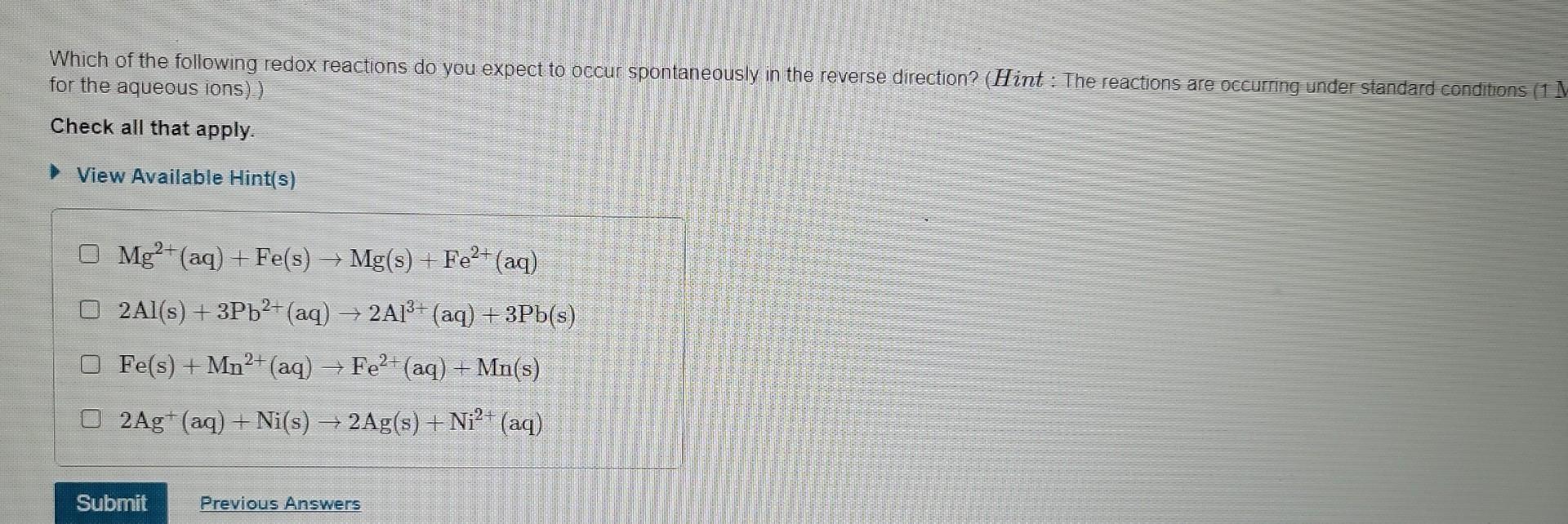
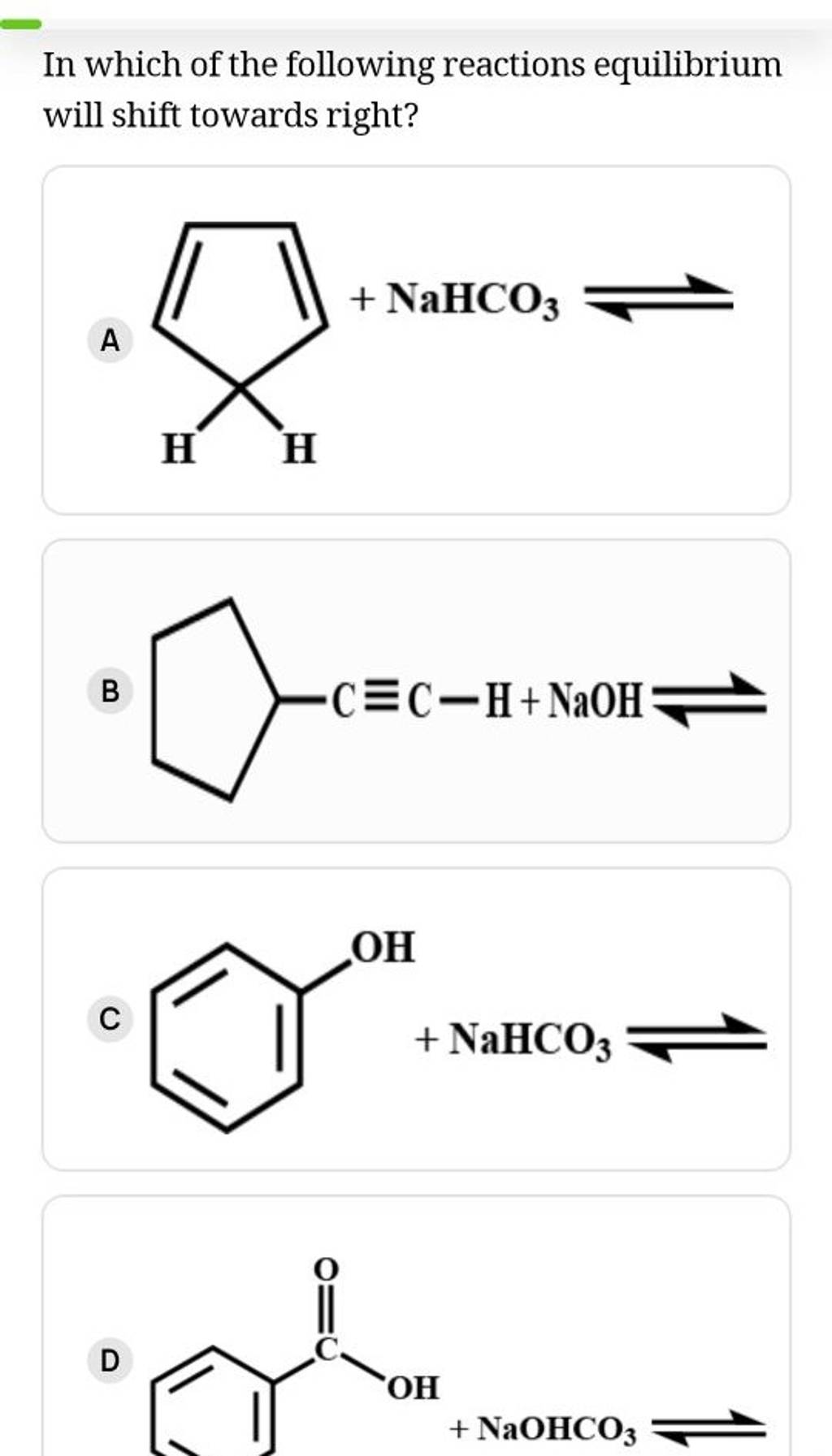
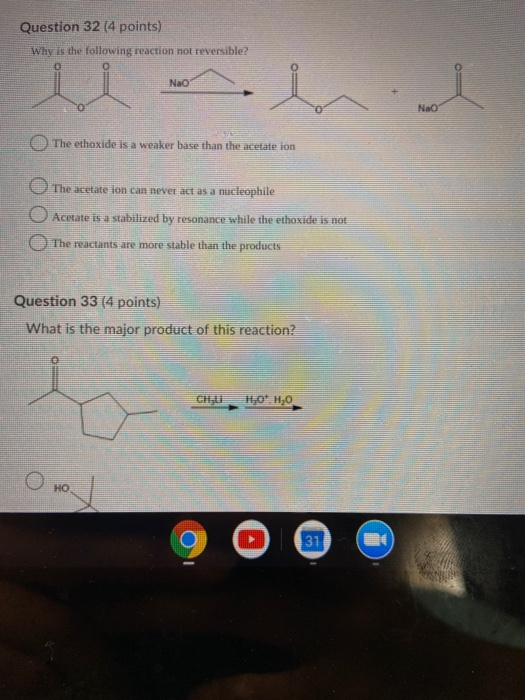
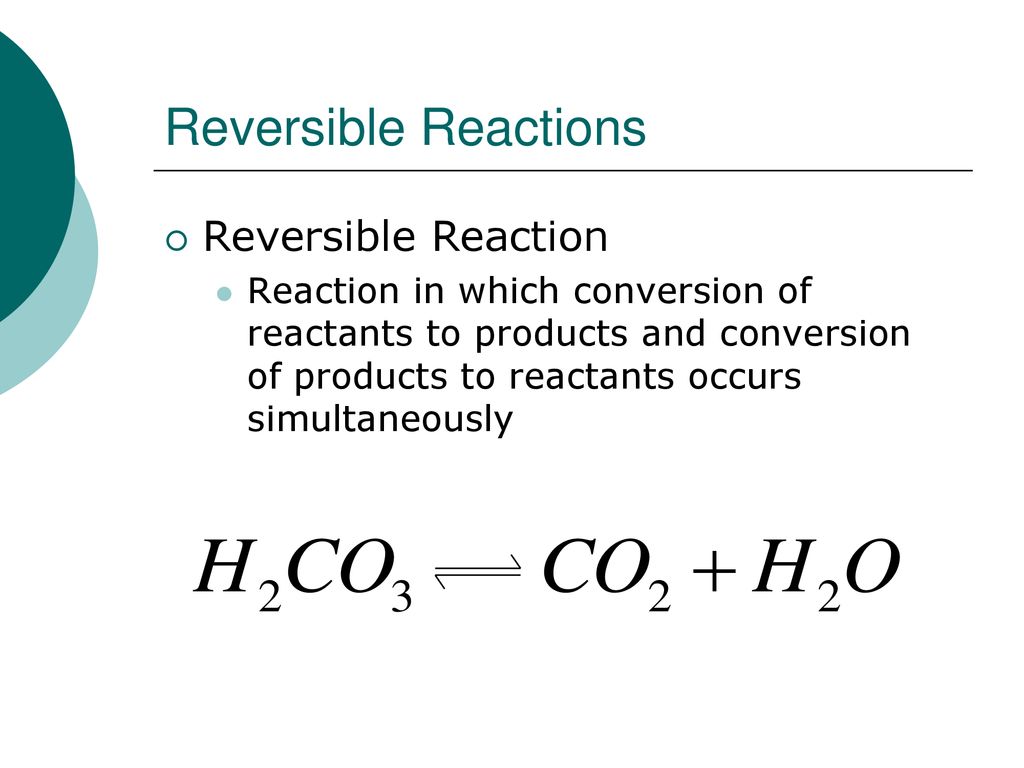Which Of The Following Reactions Is Not Reversible

The fundamental principles governing chemical reactions dictate the fate of matter, influencing everything from the formation of stars to the metabolism within our own bodies. While many reactions dance back and forth in a dynamic equilibrium, others march forward with relentless finality, forever altering the reactants into products. Understanding this distinction – discerning reversible reactions from their irreversible counterparts – is paramount to countless scientific and technological pursuits.
This article will delve into the crucial differences between reversible and irreversible chemical reactions. We will explore the defining characteristics of each, and examine the factors that determine reaction directionality. Further, we will identify key examples of irreversible reactions, illustrating their significance and implications across various scientific fields.
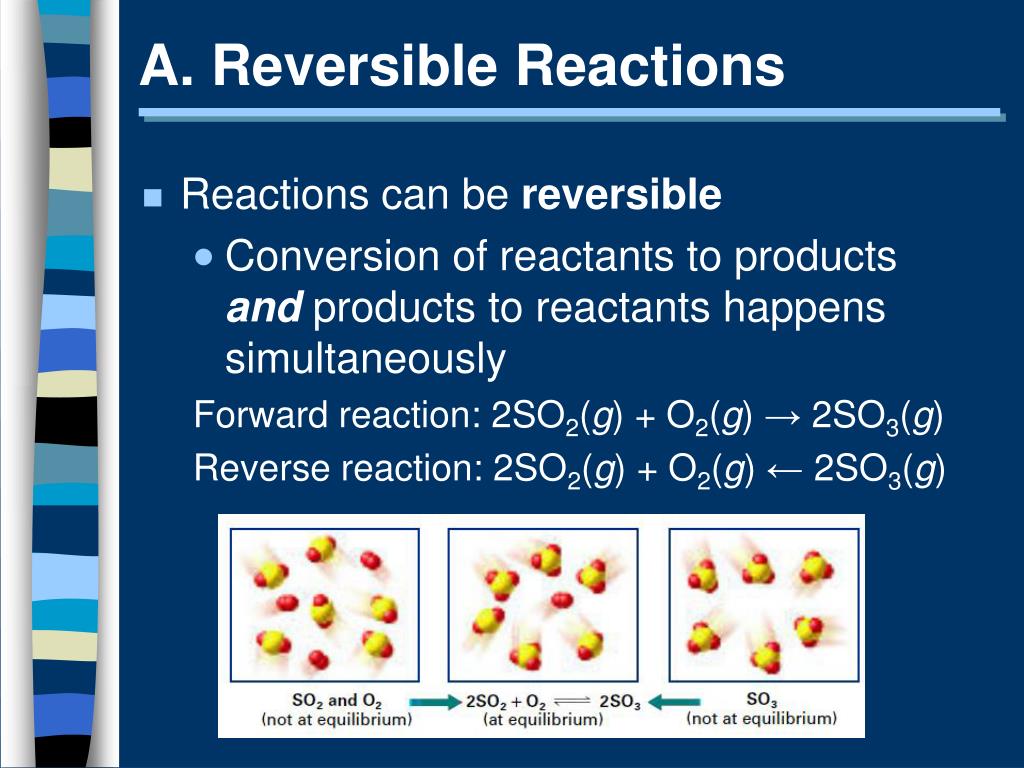
Reversible Reactions: A Balancing Act
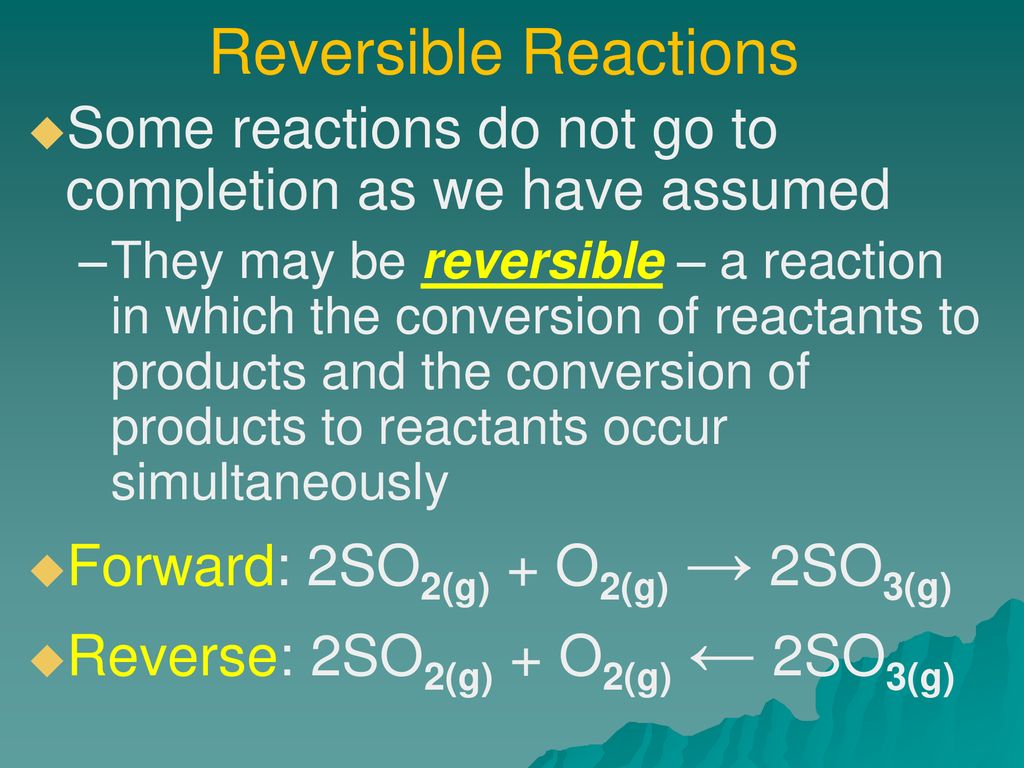
Reversible reactions, as the name suggests, are chemical processes that can proceed in both forward and reverse directions. Reactants transform into products, but simultaneously, products revert back to reactants. This dynamic interplay continues until a state of chemical equilibrium is achieved.
At equilibrium, the rates of the forward and reverse reactions are equal. The concentrations of reactants and products remain constant, though the reaction itself hasn't ceased. Le Chatelier's Principle dictates how equilibrium will shift when conditions like temperature, pressure, or concentration are altered.
Examples of Reversible Reactions
A classic example is the Haber-Bosch process, used for industrial ammonia synthesis. Nitrogen gas (N2) and hydrogen gas (H2) combine to form ammonia (NH3), but the reaction also proceeds in reverse, breaking down ammonia into its constituent elements. Another example includes the esterification of an alcohol and a carboxylic acid to form an ester and water, this can be reversed through hydrolysis.
Carbon dioxide dissolving in water is also reversible. Carbon dioxide, essential for plant life, reacts with water to produce carbonic acid. The dissolution of carbon dioxide in the ocean involves complex reversible reactions affecting oceanic pH.
Irreversible Reactions: The Point of No Return
In contrast to their reversible counterparts, irreversible reactions proceed in only one direction, effectively going to completion. Once reactants have been transformed into products, the reverse reaction is negligible or non-existent under the given conditions. This unidirectionality is often driven by the formation of a very stable product or the escape of a product from the reaction system.
Irreversible reactions release a considerable amount of energy as heat, forming stable products, or have a product constantly being removed from the system. The changes in temperature, pressure or concentration will not easily revert back to the reactants.
Characteristics of Irreversible Reactions
One key characteristic is a large negative change in Gibbs free energy (ΔG), indicating a spontaneous and energetically favorable forward reaction. The large magnitude of this energy change renders the reverse reaction highly improbable. The rate of the reverse reaction is typically so slow that it can be considered negligible.
Another indicator is the formation of a precipitate, gas, or other product that effectively removes itself from the reaction mixture. This shift pushes the reaction further towards completion, hindering the reverse process.
Identifying Irreversible Reactions: Examples and Implications
Combustion reactions are prime examples of irreversible processes. The burning of wood, gasoline, or natural gas releases heat and produces stable products like carbon dioxide and water.
The neutralization of a strong acid with a strong base is also typically considered irreversible. The reaction produces water and a salt, and the reverse reaction requires substantial energy input.
Explosions involving energetic materials such as dynamite are irreversible reactions. The rapid decomposition of reactants generates a large volume of gases and a tremendous release of energy.
The decay of radioactive isotopes is always an irreversible process. A radioactive atom decays spontaneously into different atoms, releasing particles and energy.
Distinguishing Reversible from Irreversible: A Matter of Context
The distinction between reversible and irreversible reactions is not always absolute, it's context-dependent. Some reactions can be considered reversible under certain conditions (e.g., high temperature and pressure) but irreversible under others (e.g., room temperature and standard pressure).
The categorization depends on the reaction conditions, the presence of catalysts, and the relative stability of the reactants and products. Therefore, careful analysis of experimental data and thermodynamic properties is crucial.
Conclusion: Understanding Reaction Directionality
The ability to differentiate between reversible and irreversible reactions is crucial in many scientific and technological domains. From designing chemical processes to predicting environmental outcomes, understanding reaction directionality allows scientists and engineers to control and optimize chemical transformations.
The identification of irreversible reactions is particularly important for hazard assessment, waste management, and the development of new materials. Ongoing research continues to refine our understanding of reaction dynamics, blurring the lines between reversibility and irreversibility and enabling the development of novel catalytic and synthetic strategies.