Which Phase Of Water Is Densest
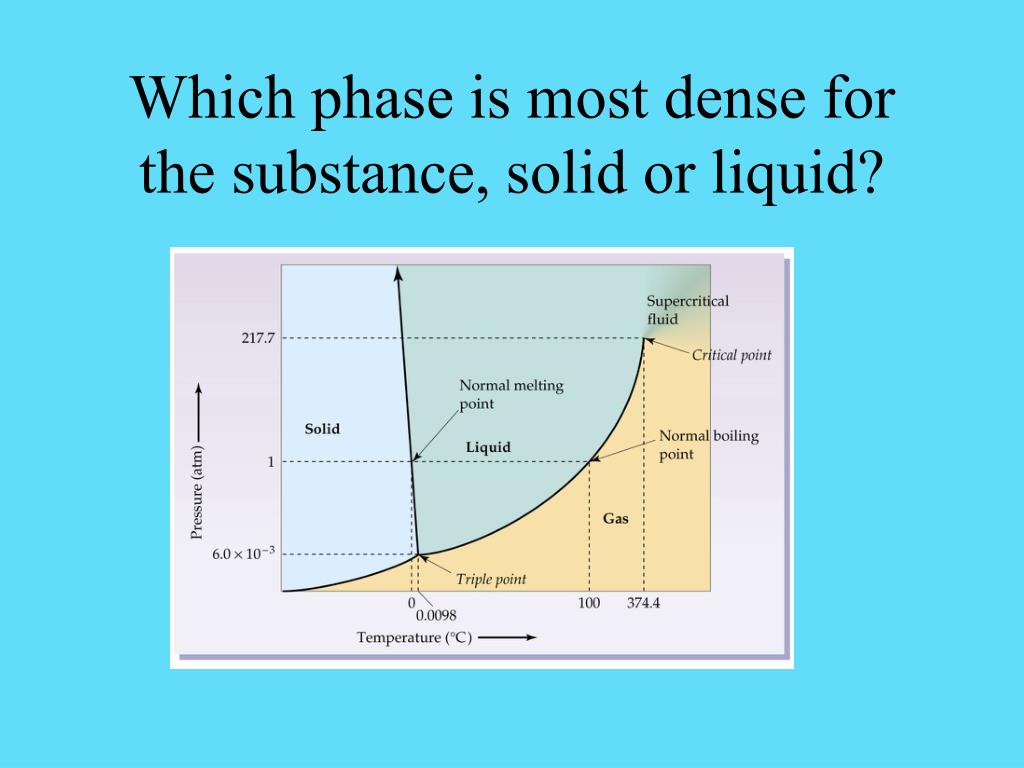
For decades, the simple question of which phase of water is densest has been a staple of science education, often answered with the seemingly straightforward "liquid water." However, the reality is far more nuanced, with recent scientific inquiry revealing surprising complexities in water's behavior under varying conditions.
This article delves into the surprising truth about water density, exploring how factors like temperature and pressure influence the arrangement of water molecules and ultimately determine its densest phase. Understanding these intricacies is crucial, not only for academic purposes but also for various practical applications, ranging from climate modeling to industrial processes.
The Conventional Wisdom: Liquid Water as the Densest Phase
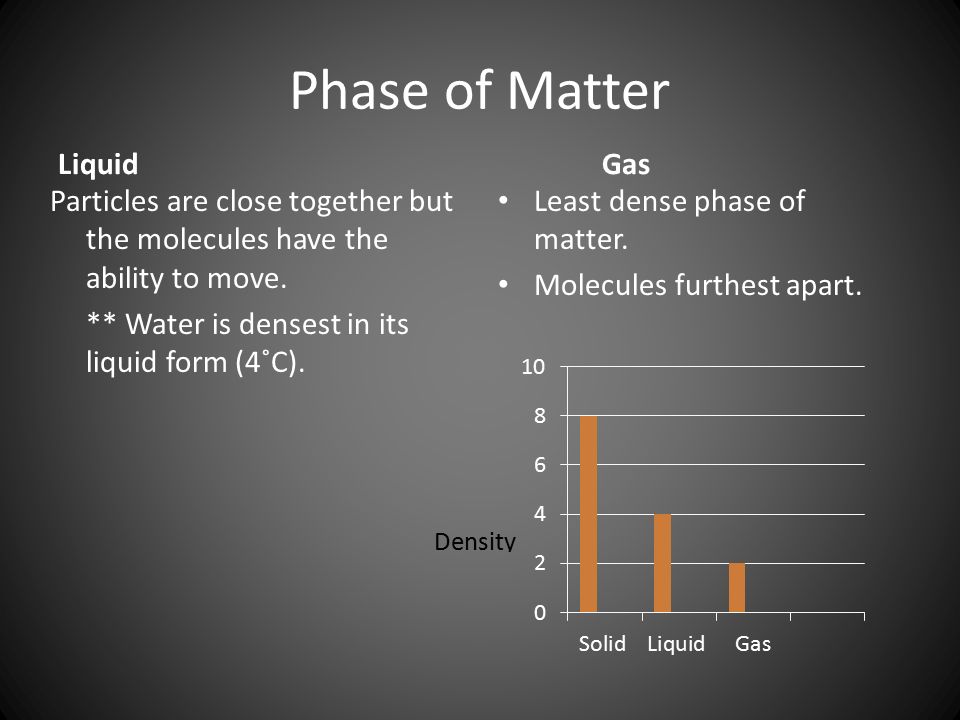
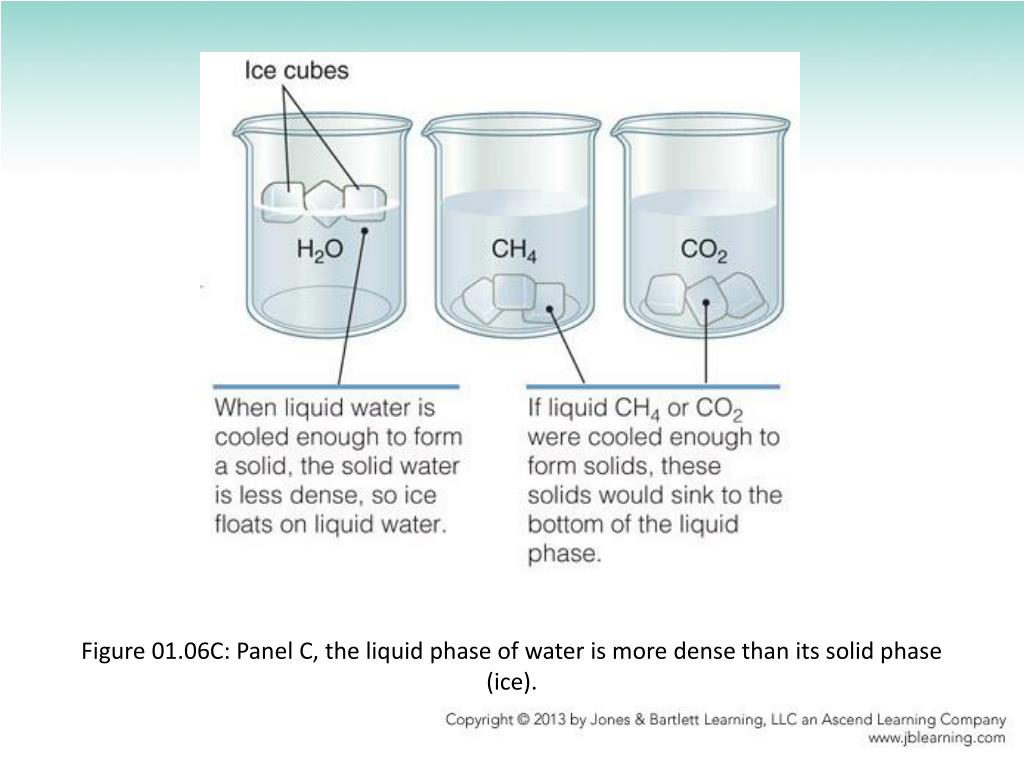
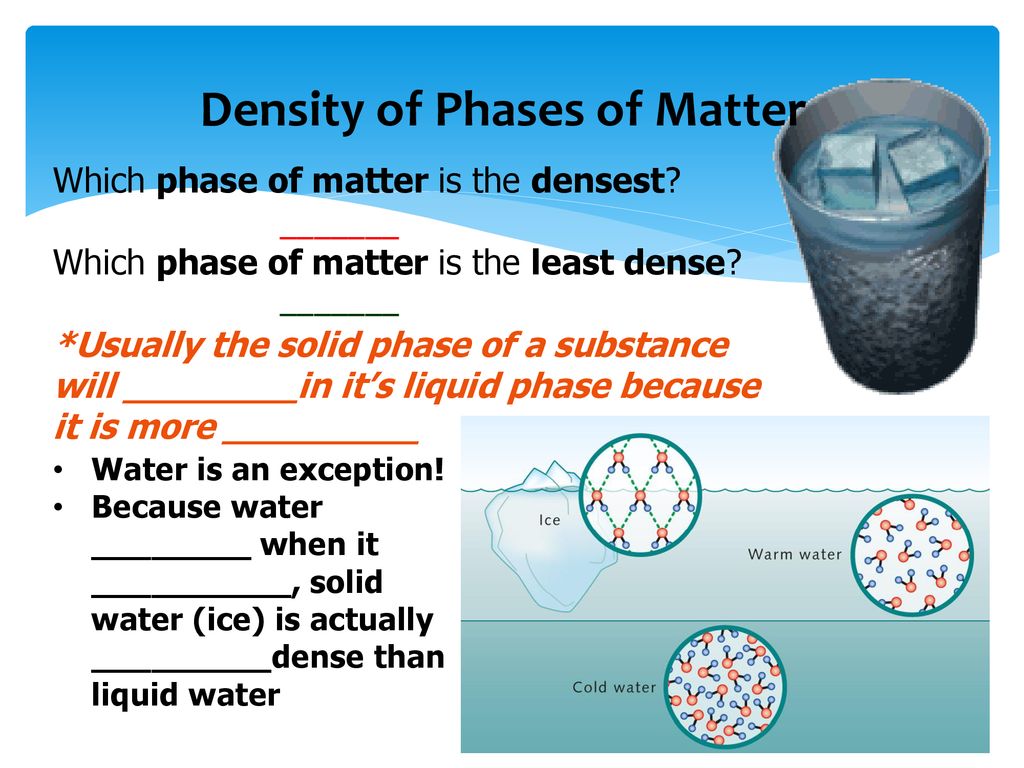
Traditionally, it's been taught that liquid water reaches its maximum density at approximately 4 degrees Celsius (39.2 degrees Fahrenheit). This anomaly is attributed to the unique hydrogen bonding network within water molecules.
As water cools from higher temperatures, its molecules pack more closely together, increasing its density. However, below 4°C, hydrogen bonds begin to dominate, forcing the molecules into a more open, crystalline-like structure, leading to a decrease in density as it approaches freezing.
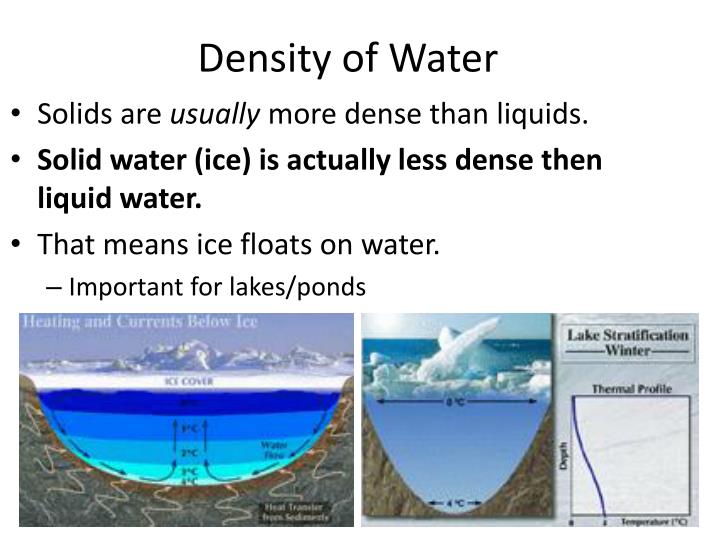
This explains why ice floats – a crucial characteristic that allows aquatic life to survive in freezing climates, as the ice layer insulates the water below.
Challenging the Standard Model: Supercooled Water
Recent research has challenged this conventional understanding by exploring the properties of supercooled water. Supercooled water is liquid water that exists below its typical freezing point (0°C or 32°F) without solidifying.
Scientists have been able to study supercooled water by carefully preventing ice crystal formation using techniques like rapid cooling and containment in small droplets. These studies have revealed surprising changes in density and other properties at extremely low temperatures.
These properties depart from the expected behavior based on the 4°C density maximum.
The Role of Pressure and Different Ice Forms
The story of water density becomes even more complex when considering the impact of pressure. Water can exist in various solid forms, known as ice polymorphs, each with a distinct crystalline structure.
These different ice polymorphs are formed under varying pressure and temperature conditions. Some ice polymorphs, especially those formed under high pressure, are significantly denser than liquid water or regular ice (Ice Ih).
For example, Ice VII, a high-pressure form of ice, can be more than 1.6 times denser than liquid water at standard conditions. These high-pressure ice forms are found in the interiors of icy planets and moons.
Experimental Evidence and Research Findings
Researchers at institutions like the University of California, Berkeley and the Max Planck Institute have conducted extensive experiments on supercooled water and high-pressure ice. These experiments utilize techniques such as X-ray diffraction and neutron scattering to probe the molecular structure of water under extreme conditions.
Their findings indicate that supercooled water exhibits a density minimum at around -38°C (-36.4°F), after which the density starts to increase again. This suggests that the density of supercooled water can surpass that of liquid water at 4°C at sufficiently low temperatures.
Furthermore, research on high-pressure ice forms has revealed a wide range of densities, with some forms exceeding the density of liquid water by a significant margin. These findings highlight the importance of considering both temperature and pressure when determining the densest phase of water.
Implications for Science and Society
The nuanced understanding of water density has several important implications. Accurately modeling the behavior of water is crucial for climate prediction.
Understanding the density of water at various temperatures and pressures is essential for developing accurate climate models and predicting the impacts of climate change. The behavior of water in these models directly affects predicted ocean currents and ice cap behavior.
Furthermore, the properties of high-pressure ice are relevant to understanding the geology and composition of icy bodies in our solar system, such as Europa and Enceladus. Finally, this knowledge can also improve industrial processes that involve water at extreme temperatures or pressures.
Conclusion: A Complex and Ever-Evolving Understanding
While liquid water at 4°C is often presented as the densest phase, the reality is considerably more complex. Supercooled water at very low temperatures and high-pressure ice forms can both be denser than liquid water under standard conditions.
This understanding is constantly evolving as scientists continue to probe the properties of water under extreme conditions. Future research will undoubtedly reveal even more surprising and fascinating aspects of this seemingly simple, yet remarkably complex, substance.
Ultimately, a deeper appreciation of water's behavior is crucial for addressing some of the most pressing challenges facing our planet, from climate change to resource management.










..jpg)


