Are Steel And Iron The Same

The terms iron and steel are often used interchangeably, leading to widespread confusion about their true nature. From skyscrapers that pierce the clouds to the humble cutlery in our drawers, both materials underpin much of modern civilization. But are they, in fact, one and the same? The answer, decisively, is no.
This article delves into the fundamental differences between iron and steel, exploring their unique compositions, properties, manufacturing processes, and diverse applications. Understanding these distinctions is crucial for engineers, manufacturers, and anyone seeking a clearer grasp of the materials that shape our world. Let’s dissect these vital metals, revealing the science behind their strengths and limitations.
Defining the Basics: Iron's Pure Form
Iron, in its purest form, is a metallic element found abundantly in the Earth's crust. It is rarely found in a pure state naturally; it usually exists as iron ore, combined with other elements like oxygen.
To obtain iron, these ores undergo a reduction process, typically in a blast furnace. The result is pig iron, which contains a high percentage of carbon, making it brittle and unsuitable for many structural applications.
Steel: Iron's Enhanced Alloy
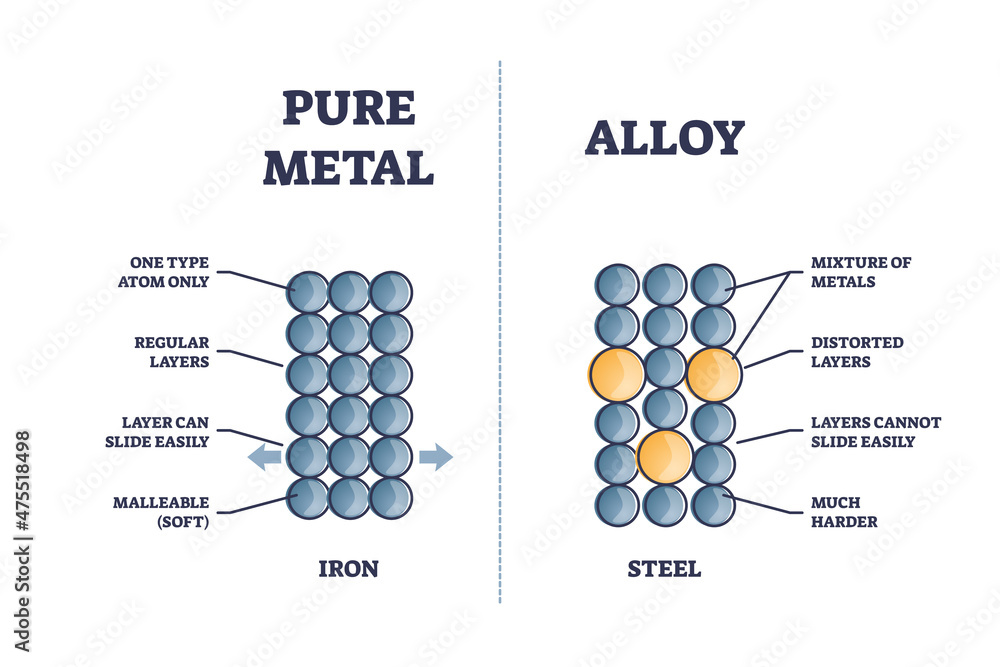
Steel, on the other hand, is not a pure element but an alloy, meaning it is a mixture of metals. It’s primarily composed of iron, but with a controlled amount of carbon – typically between 0.002% and 2.14% by weight – added to it.
This seemingly small addition of carbon dramatically alters the properties of the base metal. The controlled inclusion of other elements, such as manganese, chromium, nickel, and molybdenum, allows steel to be tailored for a wide range of specific applications.
The Carbon Conundrum: Properties and Performance
The key difference between iron and steel lies in the carbon content. This seemingly minor difference profoundly impacts their properties.
Increasing the carbon content in iron generally increases its hardness and strength, but also its brittleness. Too much carbon makes the material prone to fracture under stress.
Steel's controlled carbon content provides a superior balance between strength, ductility (ability to deform without breaking), and weldability compared to iron. Different grades of steel offer a spectrum of these properties, allowing engineers to select the optimal material for specific requirements.
Manufacturing Processes: From Ore to Application
The journey from iron ore to finished product differs significantly between the two materials. Iron production traditionally involves smelting iron ore in a blast furnace to produce pig iron, which is then refined.
Steel manufacturing builds upon this process. The excess carbon in pig iron is removed, and precise amounts of other elements are added in a controlled environment, often using processes like the basic oxygen furnace or electric arc furnace.
These processes allow for precise control over the final composition and properties of the steel.
Applications: A World of Difference
Due to its properties, pure iron has limited structural applications. Wrought iron, with a very low carbon content, was historically used for decorative ironwork, fences, and some structural components before the widespread adoption of steel.
However, steel's superior strength, ductility, and versatility make it the material of choice for a vast array of applications. These include construction (buildings, bridges), transportation (automobiles, trains, ships), manufacturing (machinery, tools), and energy (pipelines, wind turbines).
According to the World Steel Association, global steel demand remains strong, driven by infrastructure development and manufacturing growth in emerging economies.
Variations in Steel: A Spectrum of Alloys
The term "steel" encompasses a wide range of alloys, each with its own unique characteristics. Carbon steel, the most common type, contains primarily iron and carbon.
Alloy steels contain additional elements, such as chromium, nickel, or molybdenum, to enhance specific properties. Stainless steel, renowned for its corrosion resistance, contains a significant amount of chromium.
These different types of steel are carefully engineered to meet the demands of various industries and applications. For example, high-strength low-alloy (HSLA) steels are used in automotive manufacturing to reduce weight and improve fuel efficiency.
The Future of Steel and Iron
Both steel and iron continue to play crucial roles in the modern world. The steel industry is actively exploring more sustainable production methods, including reducing carbon emissions and increasing the use of recycled steel.
Research into new steel alloys with enhanced properties is ongoing, driven by the demands of emerging technologies. Nanotechnology, for example, holds promise for creating ultra-high-strength steels with exceptional performance.
While iron in its pure form has limited applications, it remains the fundamental building block upon which steel, the backbone of modern infrastructure, is built. Understanding their distinct characteristics is essential for informed decision-making in engineering, manufacturing, and beyond. The future of both materials hinges on innovation and sustainable practices, ensuring their continued contribution to a rapidly evolving world.