Bi 1823911 Kras G12c Inhibitor Clinical Trial
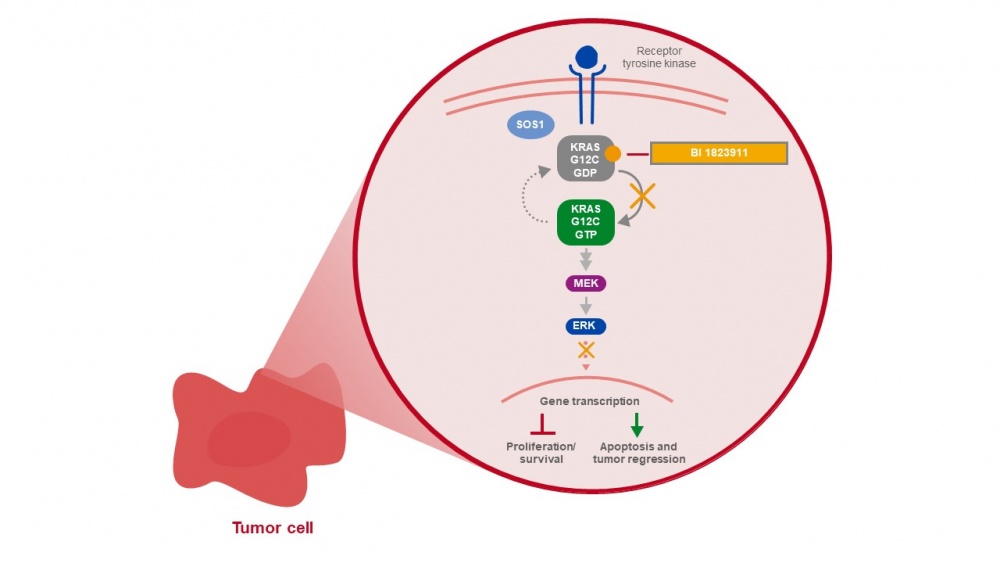
The relentless pursuit of effective cancer treatments has achieved a significant milestone with promising results emerging from clinical trials of a novel KRAS G12C inhibitor, BI 1823911. This experimental drug is generating excitement within the oncology community as it targets a notoriously challenging mutation, offering a beacon of hope for patients with specific types of tumors.
BI 1823911, developed by Boehringer Ingelheim, is an investigational oral small molecule designed to selectively and potently inhibit the KRAS G12C protein. This mutation is a driver of cancer growth in several tumor types, including non-small cell lung cancer (NSCLC), colorectal cancer (CRC), and other solid tumors. Clinical trial results, presented at recent medical conferences and in peer-reviewed publications, indicate that BI 1823911 exhibits encouraging anti-tumor activity and a manageable safety profile, potentially transforming the treatment landscape for patients with these cancers.
Understanding KRAS G12C and the Need for Targeted Therapies
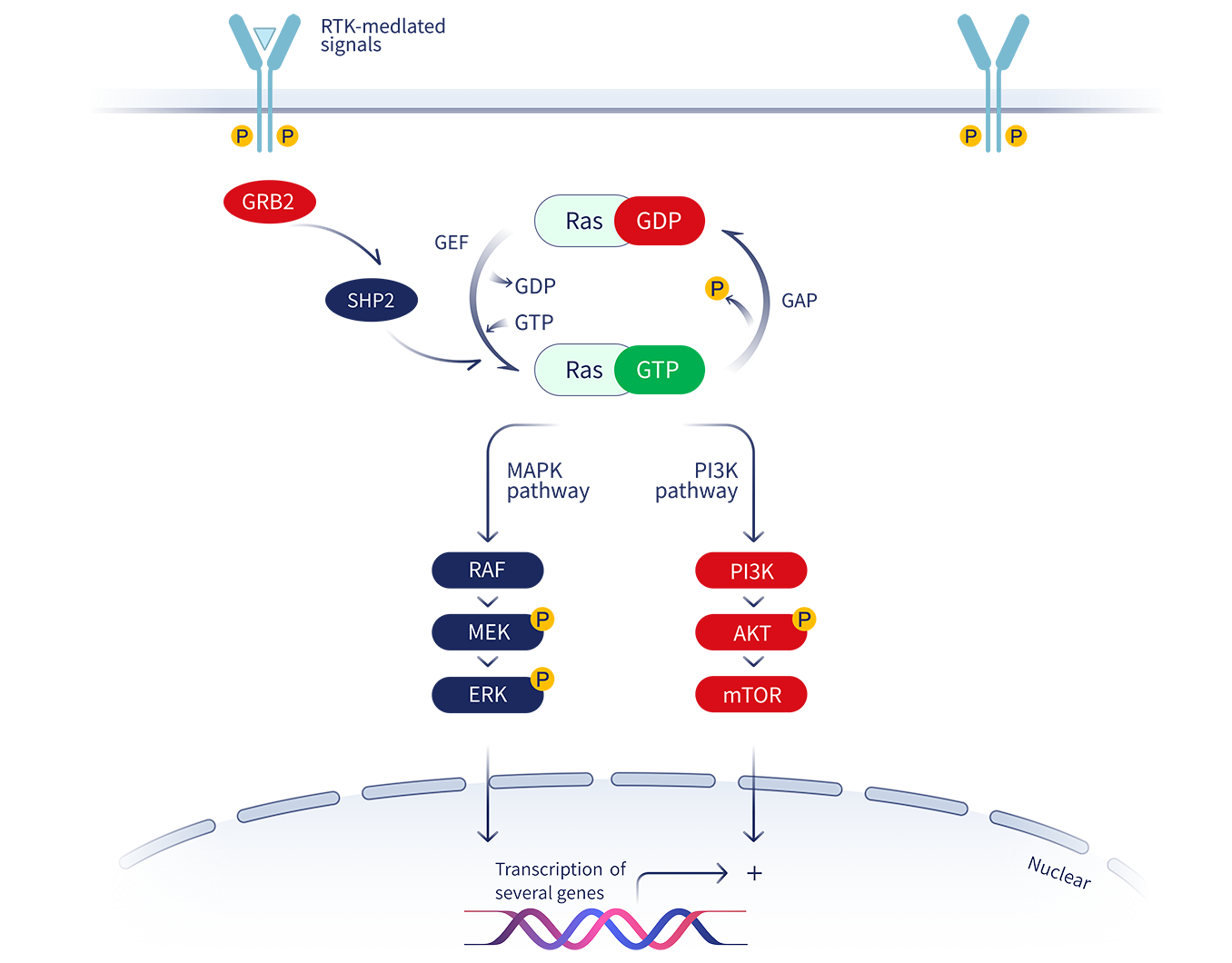
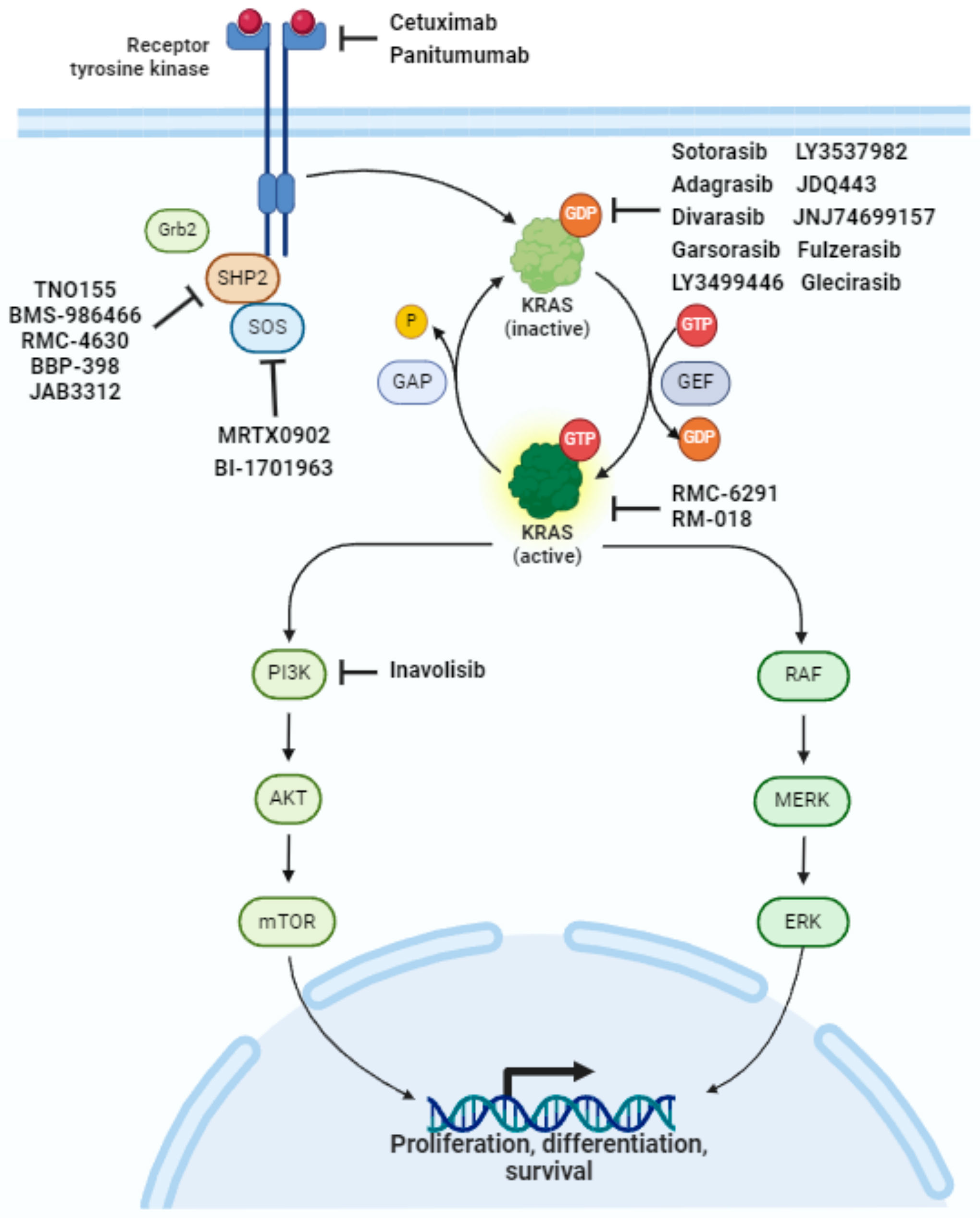
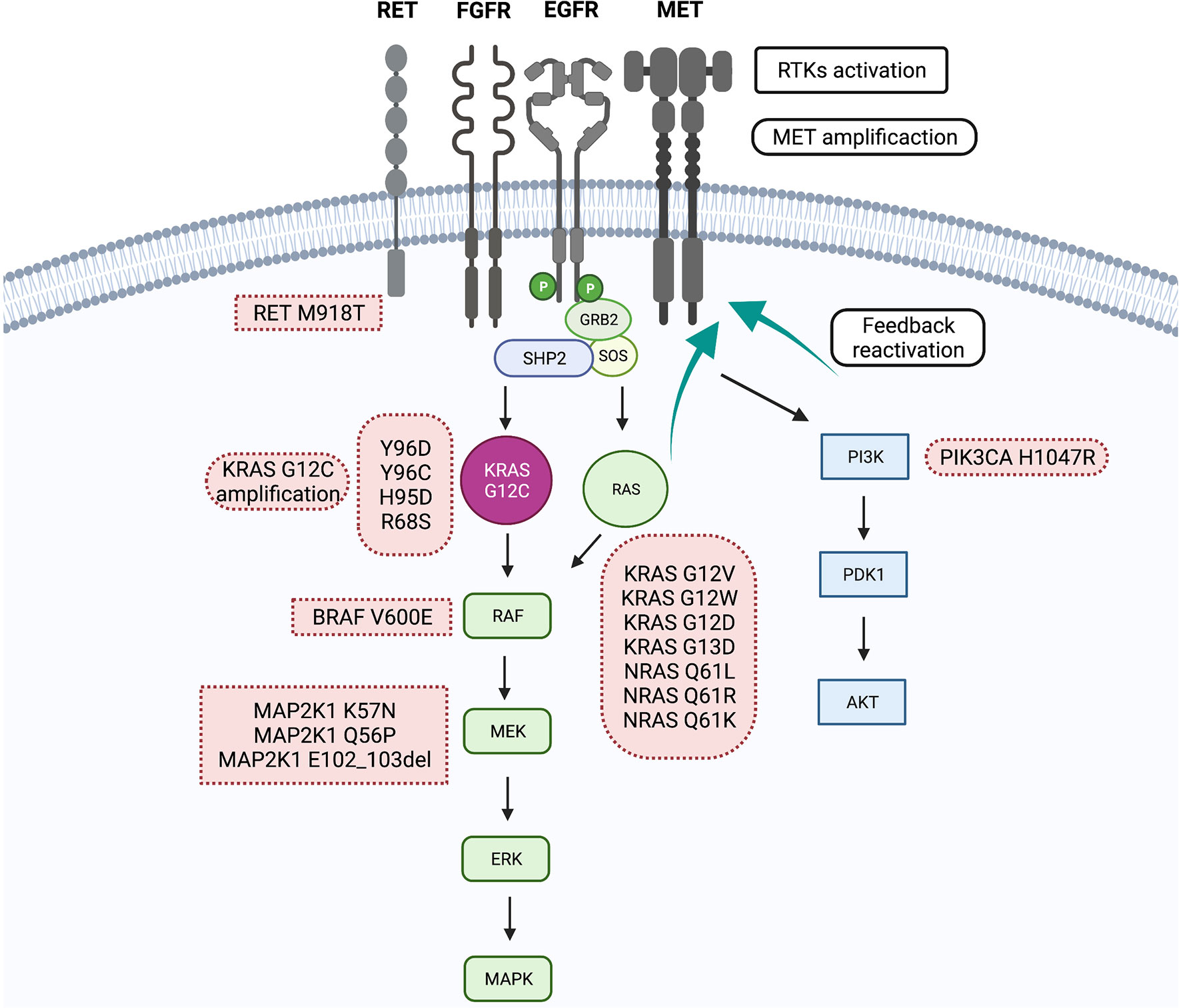
The KRAS gene is a critical component of cell signaling pathways that regulate cell growth, differentiation, and survival. Mutations in KRAS, particularly the G12C mutation, result in a perpetually activated protein, driving uncontrolled cell proliferation and tumor formation.
KRAS mutations were once considered "undruggable" due to the protein's smooth surface and lack of obvious binding pockets for traditional small molecule inhibitors. The development of KRAS G12C inhibitors like BI 1823911 represents a significant scientific breakthrough.
Clinical Trial Data: Efficacy and Safety
Early phase clinical trials evaluating BI 1823911 have focused on assessing its safety, tolerability, and preliminary efficacy in patients with advanced solid tumors harboring the KRAS G12C mutation. Initial data indicates that the drug demonstrates promising activity across various tumor types, particularly in NSCLC and CRC.
Studies have reported objective response rates (ORR) – the percentage of patients whose tumors shrink substantially – that warrant further investigation. The duration of response (DOR) – the length of time that a tumor continues to respond to treatment – also appears encouraging.
Importantly, the safety profile of BI 1823911 has been generally manageable, with the majority of adverse events being mild to moderate in severity. Common side effects observed in clinical trials include gastrointestinal disturbances, fatigue, and skin rashes.
Mechanism of Action and Differentiating Factors
BI 1823911 works by binding to the KRAS G12C protein in its inactive state, effectively locking it in that conformation and preventing it from signaling. This selective inhibition leads to reduced cell proliferation, tumor growth inhibition, and ultimately, tumor regression in some patients.
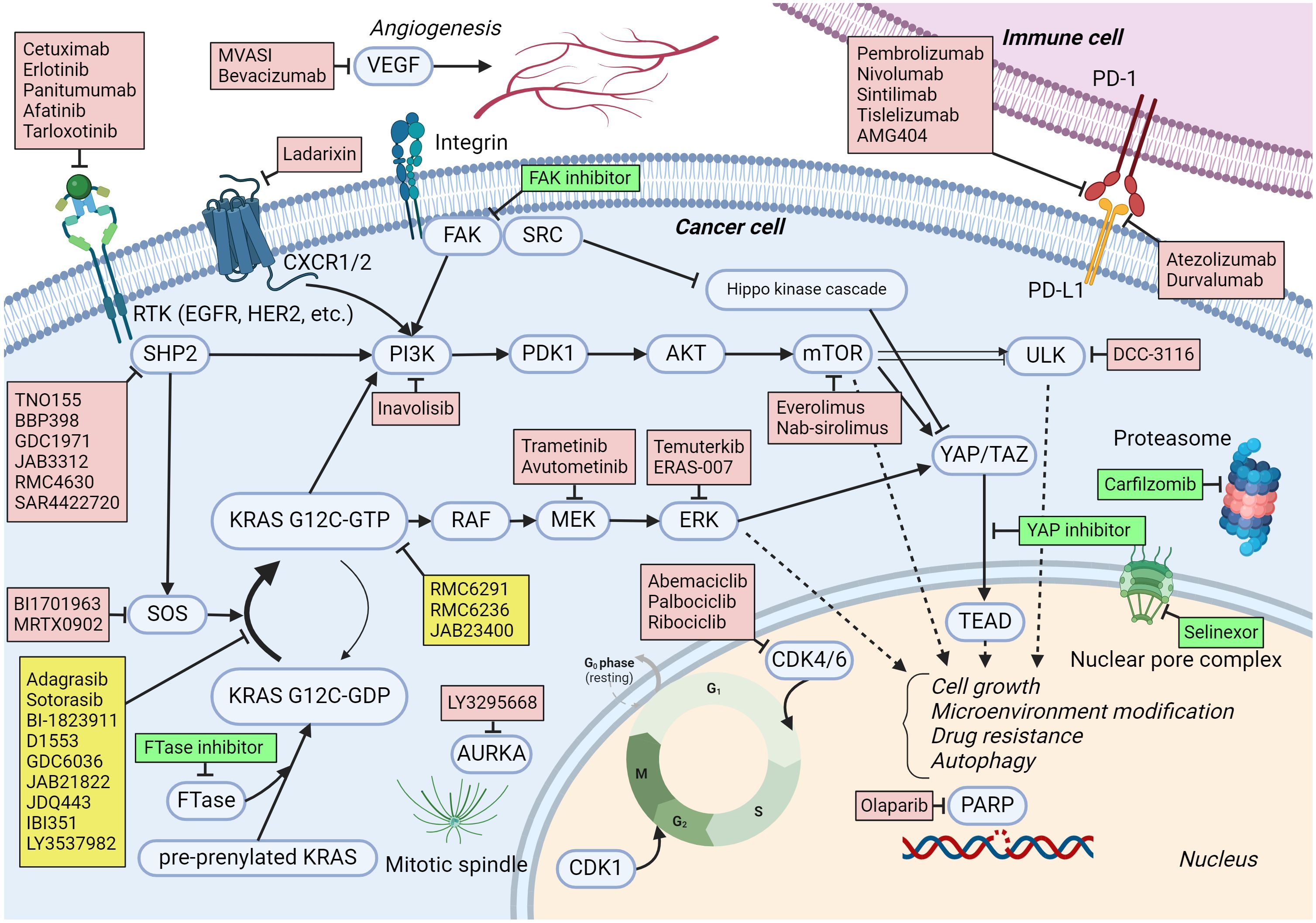
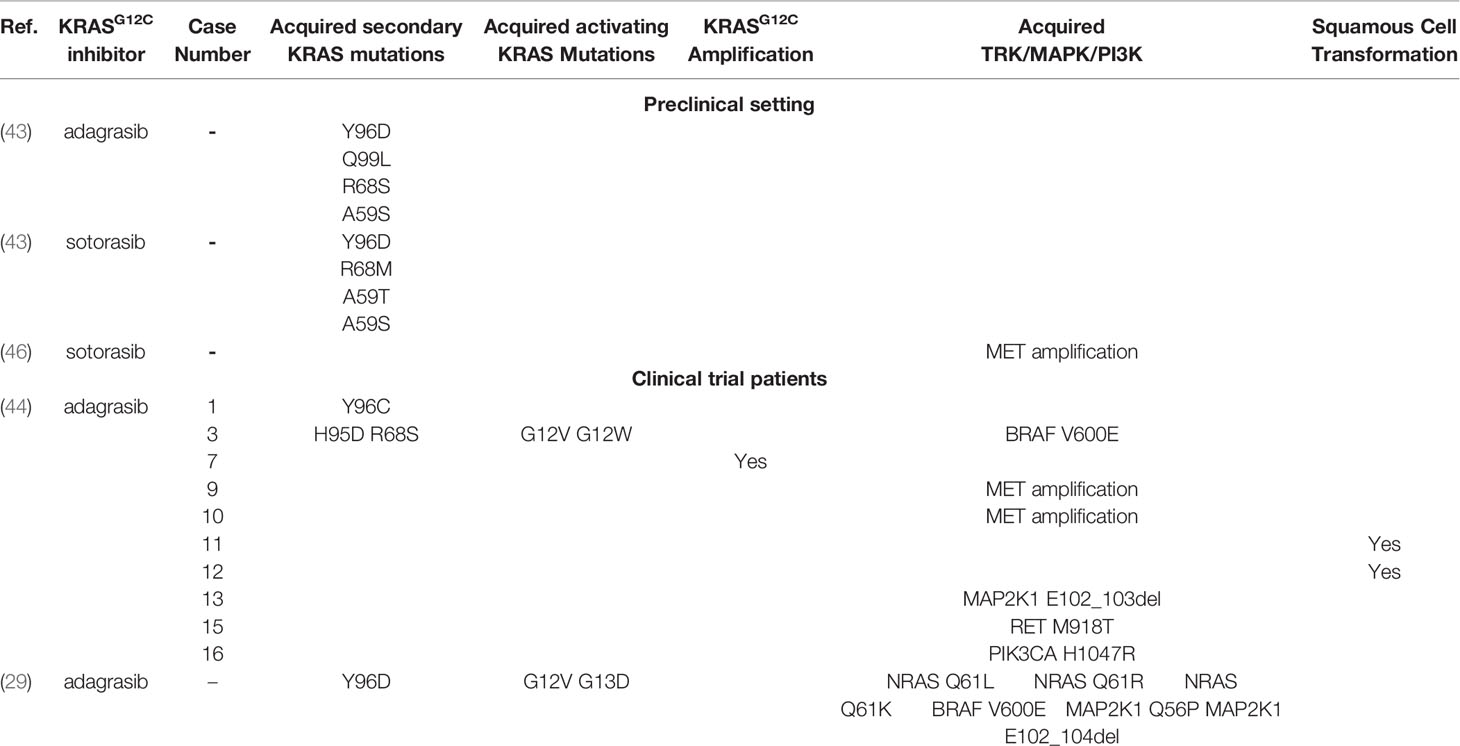
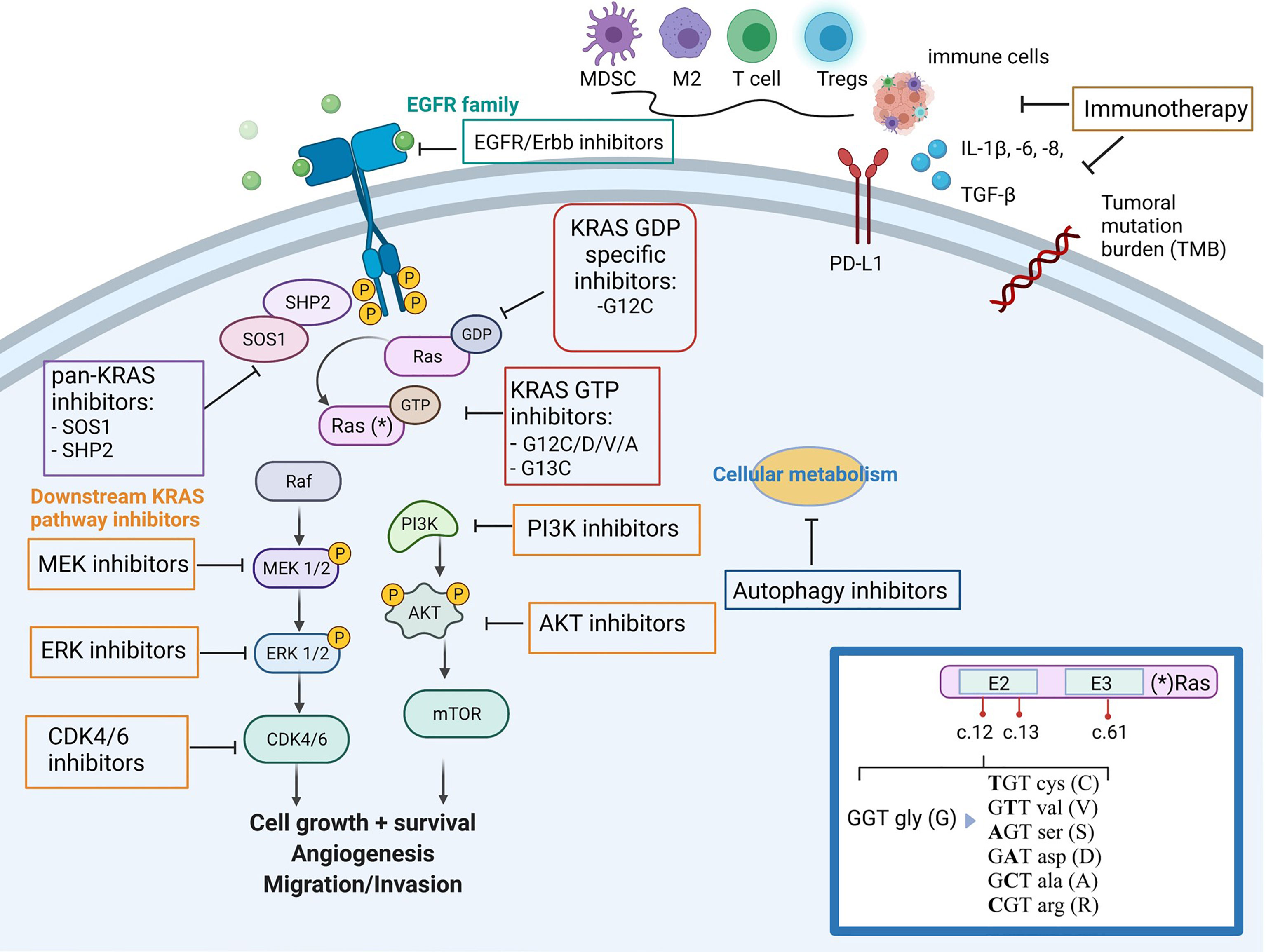
While other KRAS G12C inhibitors have already received regulatory approval, BI 1823911 possesses unique structural and pharmacological properties. These differences may translate into improved efficacy, a more favorable safety profile, or the ability to overcome resistance mechanisms that may develop with other inhibitors.
Patient Selection and Biomarker Testing
Accurate identification of patients who are most likely to benefit from BI 1823911 is crucial for optimizing treatment outcomes. Comprehensive genomic testing, including next-generation sequencing (NGS), is essential to detect the KRAS G12C mutation in tumor tissue or liquid biopsies.
Furthermore, research is underway to identify predictive biomarkers that can further refine patient selection. These biomarkers may include other genetic mutations, protein expression levels, or immune cell characteristics that correlate with response or resistance to BI 1823911.
Ongoing and Future Clinical Trials
Boehringer Ingelheim is actively conducting a broad range of clinical trials to further evaluate the safety and efficacy of BI 1823911 in various settings. These studies include monotherapy trials in patients with advanced NSCLC and CRC, as well as combination therapy trials in conjunction with other anti-cancer agents, such as chemotherapy or immunotherapy.
The company is also exploring the potential of BI 1823911 in earlier lines of therapy, with the aim of improving long-term outcomes for patients with KRAS G12C-mutated cancers. The design of these trials often incorporates adaptive features, allowing for modifications based on emerging data and patient responses.
Expert Perspectives and the Future of KRAS-Targeted Therapies
Oncologists are optimistic about the potential of BI 1823911 and other KRAS G12C inhibitors to improve the lives of patients with these challenging cancers. "The development of these targeted therapies has been a game-changer," says Dr. Emily Carter, a leading lung cancer specialist. "For years, we had limited options for patients with KRAS G12C-mutated tumors, but now we have new tools that can make a real difference."
The success of KRAS G12C inhibitors is also fueling research into therapies that target other KRAS mutations. This ongoing effort promises to expand the reach of precision medicine and improve outcomes for even more cancer patients in the future.
While BI 1823911 is still an investigational agent, the clinical trial data to date suggest that it holds significant promise as a new treatment option for patients with KRAS G12C-mutated cancers. As clinical development progresses and more data become available, BI 1823911 could potentially transform the treatment paradigm and offer a new hope for patients facing these difficult diagnoses. Boehringer Ingelheim continues to advance the clinical program for BI 1823911, with the ultimate goal of bringing this innovative therapy to patients in need.




:%0A%0AJAB-21822 (KRAS G12C inhibitor) for Colorectal Cancer.png?md=1)