Chemical Inspection & Regulation Service Limited

Global chemical regulatory compliance faces potential disruption. Chemical Inspection & Regulation Service Limited (CIRS), a key player in this sector, is under increased scrutiny.
This situation has triggered immediate concern among companies relying on CIRS for navigating complex chemical regulations worldwide, demanding swift understanding and adaptation to potential shifts in compliance strategies.
Who is CIRS?
CIRS is a consulting company providing comprehensive chemical regulatory compliance services.
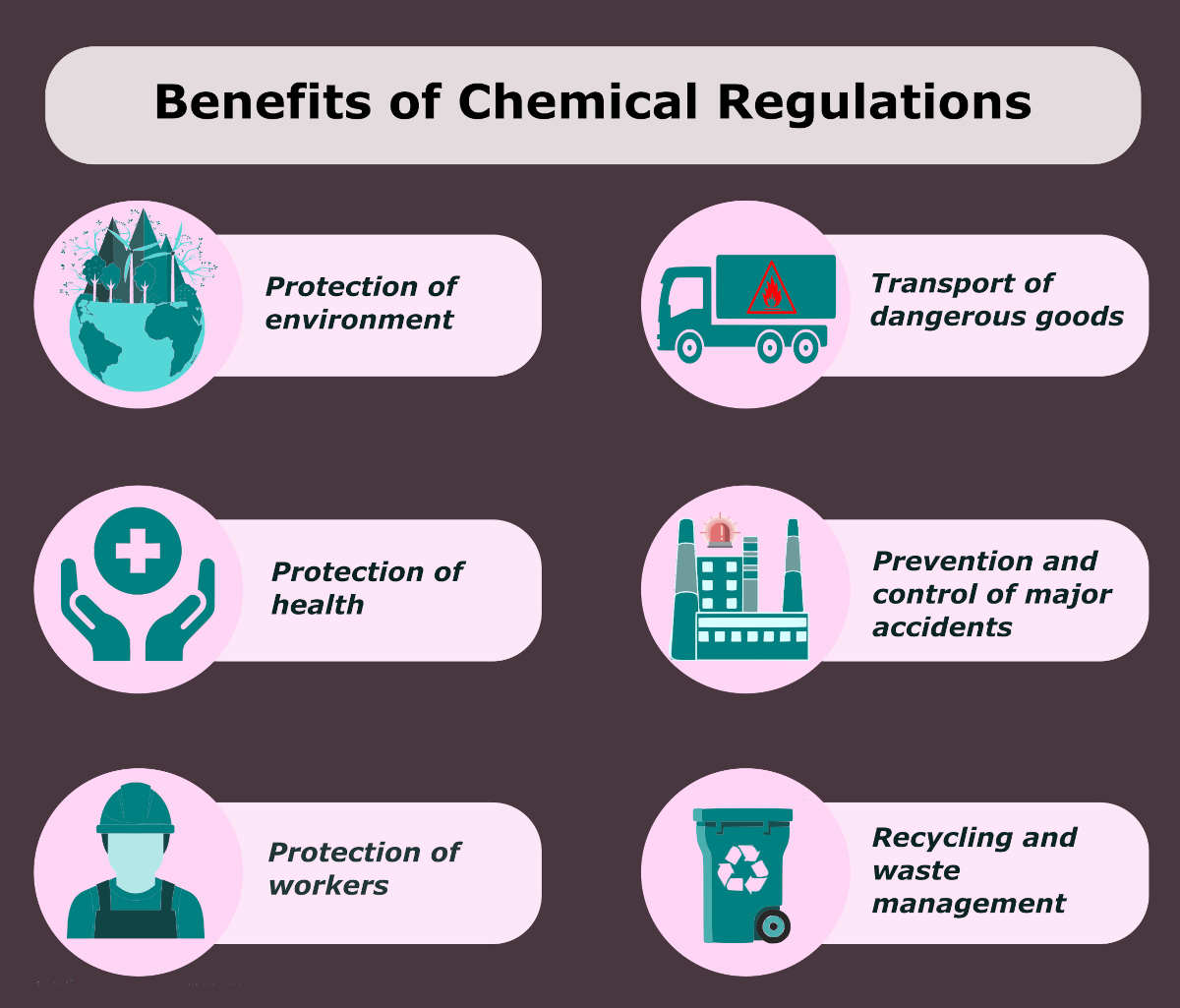
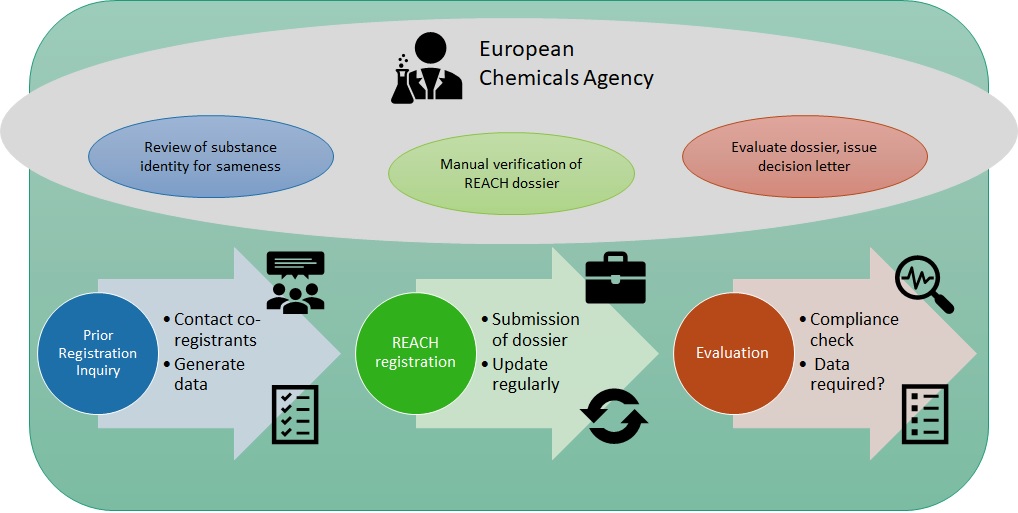
They offer expertise in areas like REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals), SDS (Safety Data Sheet) authoring, and GHS (Globally Harmonized System) implementation across various regions, including Europe, Asia, and North America.
The company has offices in multiple countries, including Ireland, China, and the United States.
What is Happening?
While specific details remain confidential due to ongoing legal and internal processes, sources indicate increased regulatory oversight on CIRS’s operations.
This situation involves examination of their compliance procedures and documentation processes.
The exact scope and nature of the scrutiny are not yet fully disclosed, leading to industry uncertainty.
Where is this Happening?
The increased regulatory oversight affects CIRS operations globally.
European and Asian operations appear to be the initial focus of the investigation.
The impact extends to any company using CIRS services to comply with regulations in these regions.
When Did This Start?
The increased scrutiny began in the third quarter of 2023, although the initial signs were subtle.
The intensity of the investigation has increased in recent weeks, with more formal inquiries and audits reported.
Information suggests the situation is actively unfolding.
How is this Impacting the Industry?
Companies relying on CIRS are now evaluating alternative compliance strategies.
Many are seeking independent verification of existing compliance documentation prepared by CIRS.
This situation may lead to delays in product registrations and market access, increased costs, and potential disruptions to supply chains.
Immediate Actions for Affected Companies
Conduct Internal Audits: Immediately review all compliance documentation provided by CIRS.
Seek Independent Verification: Engage independent consultants to verify the accuracy and completeness of existing compliance files.
Develop Contingency Plans: Identify alternative compliance pathways and providers to mitigate potential disruptions.
Potential Consequences
Possible outcomes range from minor procedural adjustments to significant operational overhauls for CIRS.
For companies using CIRS, the consequences could include the need to resubmit compliance documentation, facing regulatory penalties, and potential product recalls in severe cases.
The long-term impact on the chemical regulatory compliance landscape remains to be seen.
Next Steps and Ongoing Developments
Regulatory bodies are expected to release further statements and findings in the coming weeks.
Industry stakeholders are closely monitoring the situation for any official announcements or policy changes.
Companies should proactively engage with regulatory agencies and legal counsel to navigate this evolving landscape effectively.
This is a developing story, and updates will be provided as more information becomes available. Affected parties are urged to stay informed and take appropriate action.













.jpg)

