Diamond Is An Element Or Compound

Urgent clarification is needed: Public confusion persists regarding the fundamental composition of diamonds. Is it an element or a compound?
This article aims to definitively settle the debate surrounding diamond's classification, offering a concise explanation based on established scientific understanding and addressing the widespread misinformation.
The Definitive Answer: Diamond is an Element
Diamonds are unequivocally classified as elements. An element is a pure substance consisting of only one type of atom.
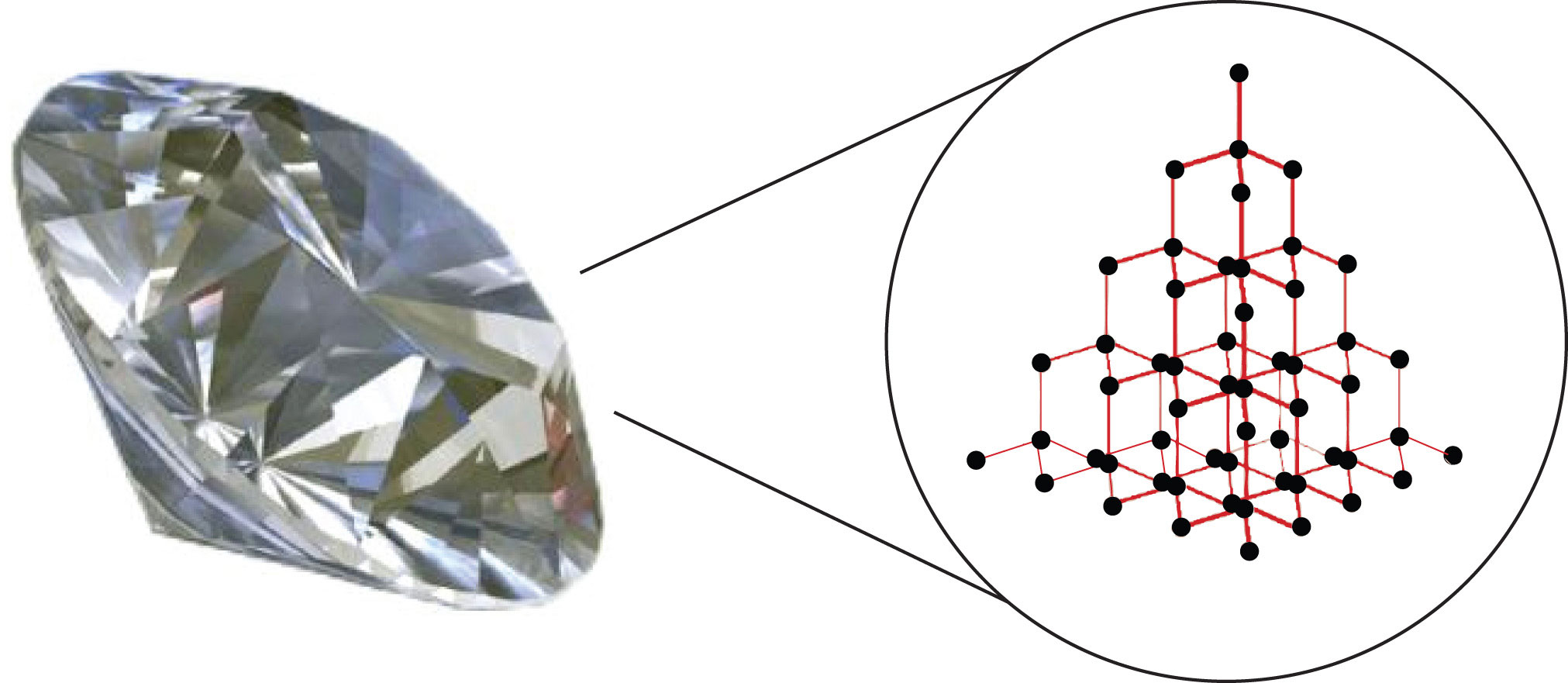
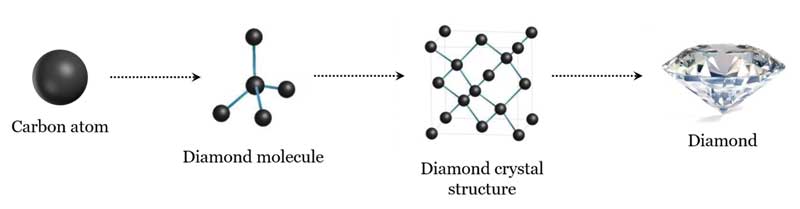
In the case of diamonds, they are solely composed of carbon (C) atoms arranged in a specific crystal structure.
Understanding Elemental Composition
Elements, such as hydrogen (H), oxygen (O), and gold (Au), are the simplest forms of matter and cannot be broken down into simpler substances by chemical means.
Compounds, on the other hand, are formed when two or more different elements are chemically bonded together, like water (H2O) or carbon dioxide (CO2).
Diamond's Unique Carbon Structure
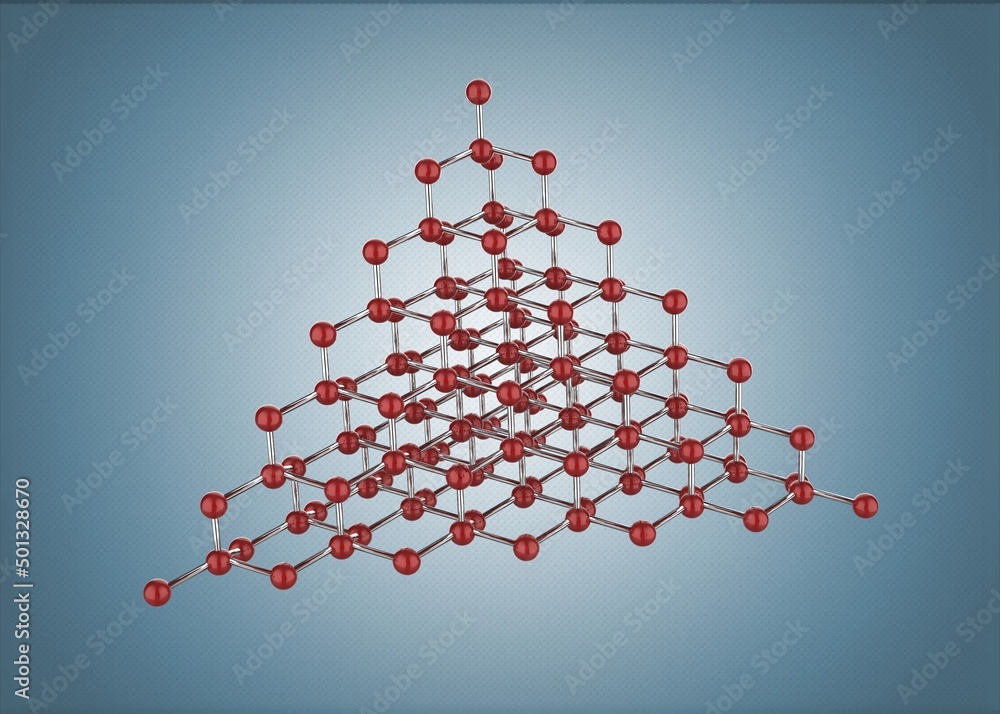
The extraordinary properties of diamonds, including their hardness and brilliance, stem from the strong covalent bonds between the carbon atoms in their tetrahedral lattice structure.
Each carbon atom is bonded to four other carbon atoms, forming a rigid, three-dimensional network.
This structure is what gives diamonds their exceptional strength and resistance to compression.
Distinguishing Diamond from Carbon-Based Compounds
While diamonds are purely carbon, many carbon-containing substances exist. These carbon-containing substances include graphite, charcoal, and organic molecules.
These substances are compounds because they contain other elements besides carbon, or the carbon atoms are arranged in a different structure (as in graphite).
Addressing Common Misconceptions
The misconception that diamonds are compounds likely arises from the fact that they can contain trace amounts of other elements, such as nitrogen or boron. However, these trace elements do not fundamentally alter the diamond's classification as a pure carbon element.
These elements are considered impurities within the carbon lattice, not constituent parts of a chemical compound.
Scientific Consensus and Validation
The scientific community universally agrees on the elemental nature of diamonds. This is based on decades of research in chemistry, physics, and materials science.
Academic sources and authoritative texts consistently classify diamonds as an element.
"Diamond is an allotrope of carbon, meaning it is a different structural form of the same element." -Leading Chemistry Textbook
Practical Implications of Diamond's Elemental Status
Understanding that diamond is an element is crucial for various applications, including materials science, gemology, and industrial processes.
Its purity and well-defined structure are essential for its use in cutting tools, abrasives, and high-tech devices.
Moving Forward: Education and Awareness
Continued efforts are necessary to disseminate accurate information about the composition of diamonds. This involves educational initiatives targeting students, consumers, and the general public.
Scientific institutions and media outlets must collaborate to address misconceptions and promote a clear understanding of diamond's elemental nature.
Further research into the properties and applications of diamonds continues. Current research explores new applications of synthetic diamonds and carbon-based materials.



![Diamond Is An Element Or Compound Examples of Elements, Compounds, and Mixtures [ANSWERED] – Dear Learners](https://dearlearners.com/wp-content/uploads/2021/02/is-diamond-an-element-compound-or-mixture_-768x480.jpg)