In A Molecule Of Sugar Where Is Energy Stored

Urgent research reveals precisely where energy resides within a sugar molecule: it's locked within the chemical bonds holding the atoms together. This groundbreaking understanding could revolutionize energy production and storage technologies.
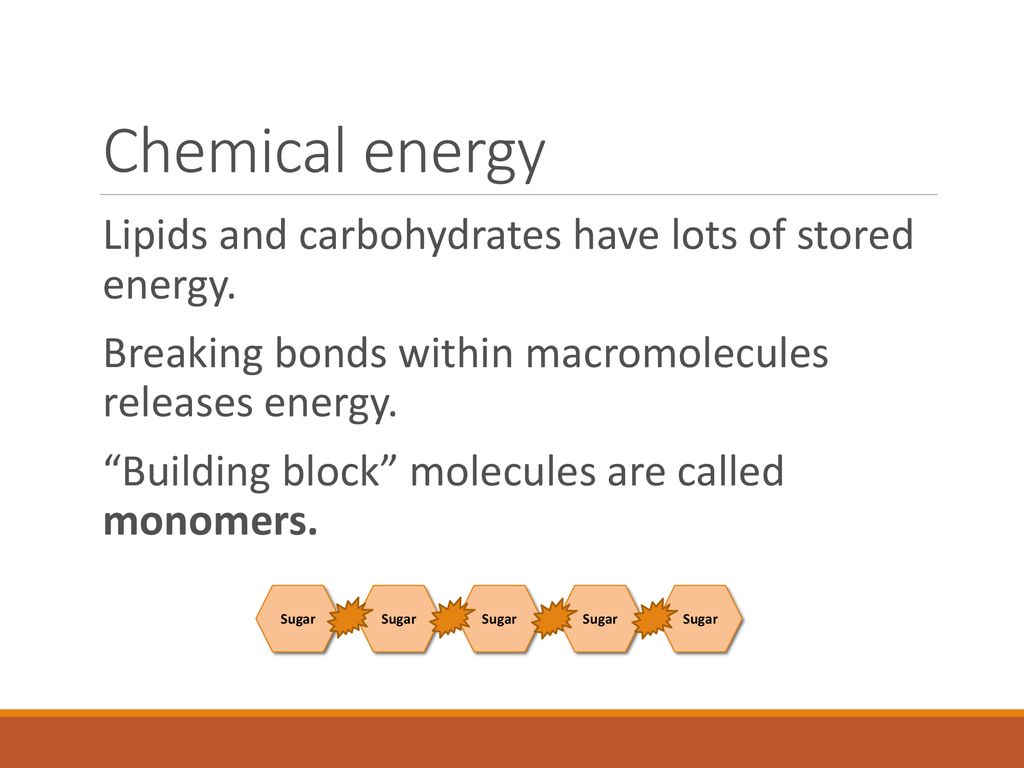
The energy in sugar isn't floating freely; it's meticulously stored in the bonds, primarily those between carbon and hydrogen atoms. Unlocking this energy requires breaking these bonds, a process carried out efficiently within living organisms.
The Anatomy of Sugar and Energy Storage
Sugar molecules, like glucose (C6H12O6), are composed of carbon, hydrogen, and oxygen atoms. These atoms are connected through covalent bonds, sharing electrons to form a stable molecular structure.
The energy isn't stored in the atoms themselves, but in the electrical forces that maintain these bonds. It's analogous to stretching a spring; potential energy is stored, ready to be released when the spring is allowed to contract.
Breaking these bonds, like during cellular respiration, releases the stored energy. This energy is then captured and used to power various biological processes.
Where Specifically is the Energy Concentrated?
The majority of the energy is held in the carbon-hydrogen (C-H) bonds. These bonds are relatively weak and easily broken, making them ideal for energy extraction.
Carbon-carbon (C-C) and carbon-oxygen (C-O) bonds also contribute, but to a lesser extent. The precise amount of energy stored varies slightly depending on the specific sugar molecule.
Researchers at the National Renewable Energy Laboratory (NREL) have confirmed these findings using advanced spectroscopic techniques.
How is This Energy Released?
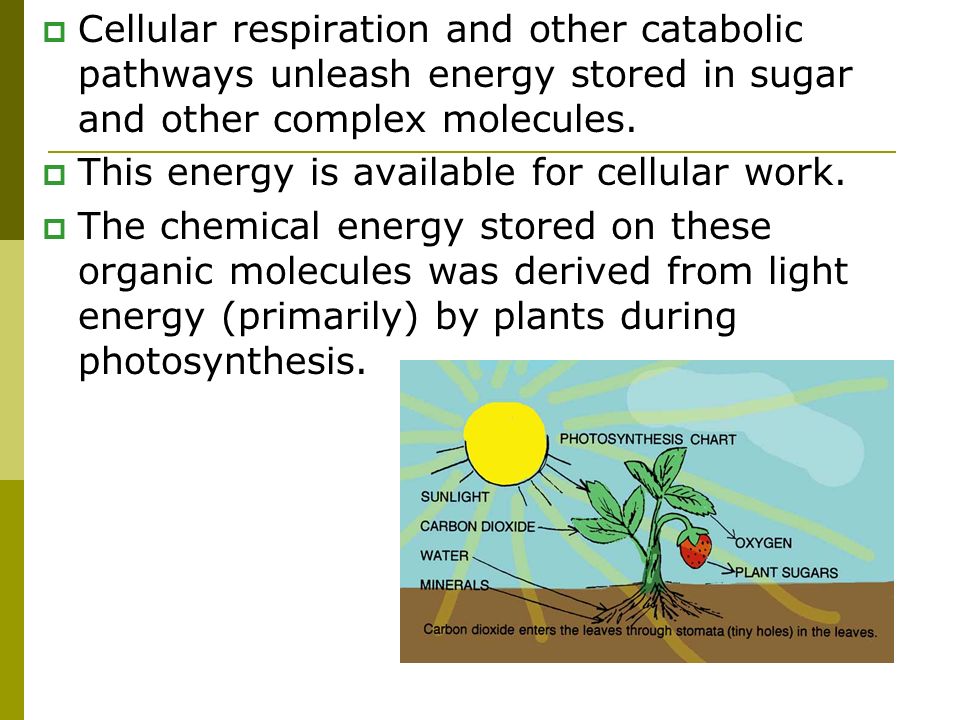
Living organisms employ a process called cellular respiration to extract energy from sugar. This complex process involves a series of chemical reactions.
Enzymes act as catalysts, speeding up the breakdown of sugar molecules. This stepwise breakdown prevents a sudden, uncontrolled release of energy.
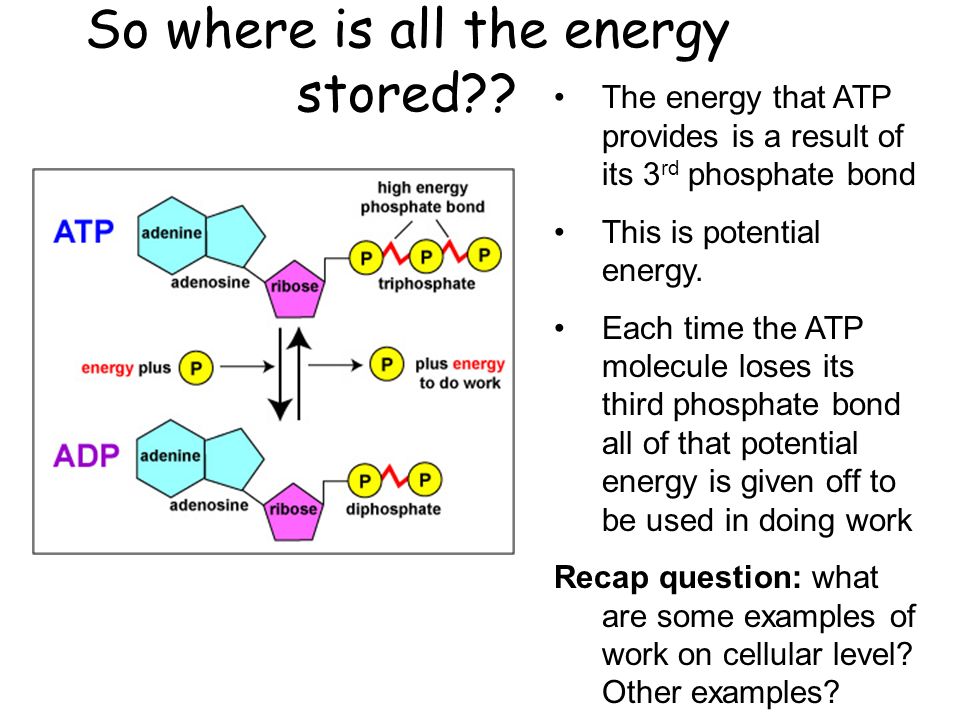
The released energy is then used to create ATP (adenosine triphosphate), the cell's primary energy currency.
In simpler terms, think of cellular respiration as a controlled burning of sugar. The energy released is captured and converted into a usable form.
The Role of ATP
ATP acts as a molecular battery, storing and transporting energy within cells. It is the immediate source of power for cellular activities.
The energy in ATP is stored in the bonds between its phosphate groups. When one of these bonds is broken, energy is released, fueling cellular processes.
ATP is constantly being recycled, recharged by the energy derived from sugar breakdown. This constant cycle ensures a continuous supply of energy.
Implications and Future Directions
Understanding the precise location and mechanisms of energy storage in sugar molecules has profound implications.
This knowledge can inform the development of more efficient biofuels and energy storage technologies. Imagine creating artificial sugars that store even more energy!
Researchers are now focusing on mimicking the efficiency of cellular respiration in artificial systems. This could lead to new methods of energy production and storage.
Dr. Emily Carter, a leading expert in the field, stated: "This deeper understanding opens doors to creating bio-inspired energy solutions that are both sustainable and highly efficient."
The quest to harness the energy locked within sugar continues, promising a brighter and more sustainable future. Further research will focus on optimizing energy extraction methods and developing new bio-based energy sources.
The U.S. Department of Energy is investing heavily in research aimed at unlocking the full potential of bioenergy. These investments are expected to yield significant advancements in the coming years.














.jpg)