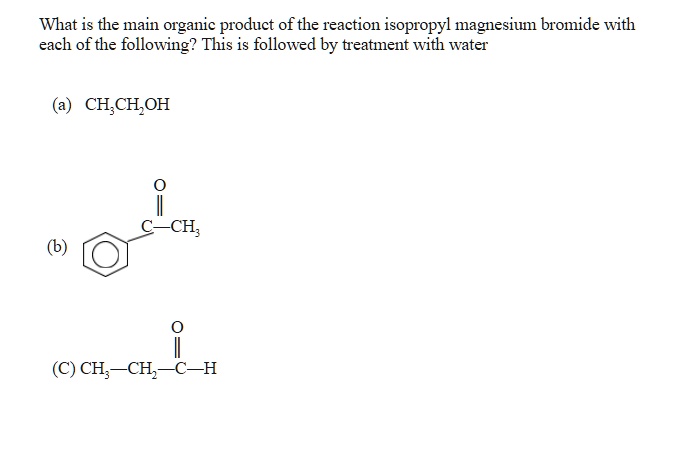
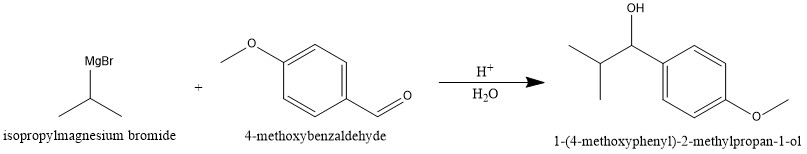
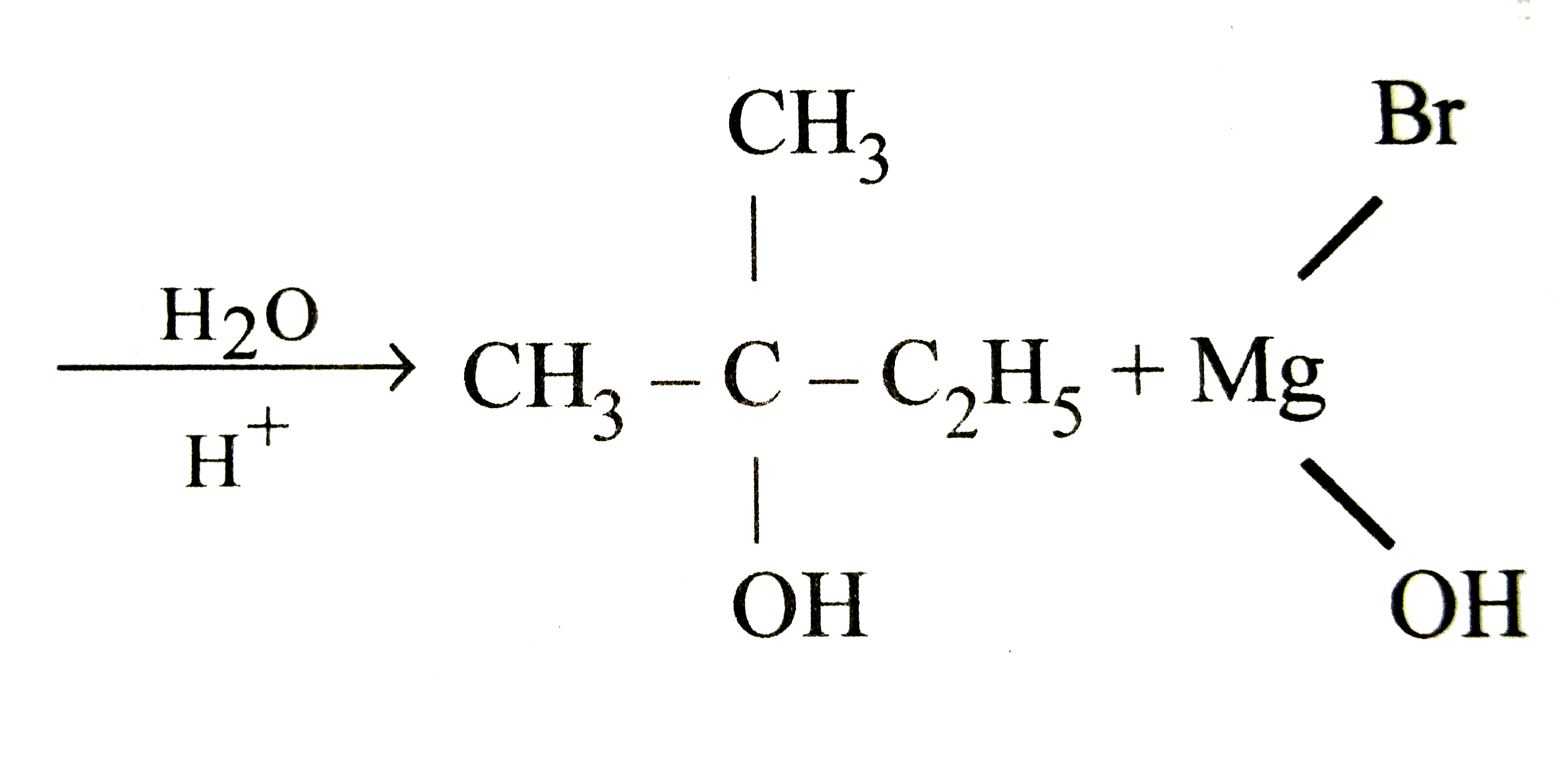Isopropyl Magnesium Bromide With Water

A highly reactive and potentially explosive situation has been documented involving the combination of Isopropyl Magnesium Bromide (iPrMgBr) and water. Immediate safety precautions are paramount for anyone handling or working near these substances.
This incident highlights the critical importance of understanding the hazardous reactions associated with Grignard reagents, particularly when exposed to water or protic solvents. Strict adherence to safety protocols is crucial to prevent severe consequences.
Immediate Danger: iPrMgBr + H2O
What: Isopropyl Magnesium Bromide (iPrMgBr), a Grignard reagent commonly used in organic synthesis, reacts violently with water (H2O). The reaction produces flammable gases and significant heat, posing a fire and explosion hazard.
Who: This hazard affects chemists, lab technicians, researchers, and anyone working with or storing these chemicals. Improper handling poses a significant risk of injury or property damage.
Where: This reaction is a concern in any laboratory setting, research facility, or chemical storage area where both iPrMgBr and water are present. Incidents have been documented in both academic and industrial laboratories.
When: The reaction occurs immediately upon contact between iPrMgBr and water. Even trace amounts of moisture can initiate the reaction.
How: The mechanism involves the rapid protonation of the Grignard reagent by water, releasing a hydrocarbon gas (propane in this case) and forming magnesium hydroxide bromide. This is an exothermic reaction, releasing substantial energy as heat.
Confirmed Incidents and Safety Concerns
Multiple laboratory incidents have been reported involving unintended contact between Grignard reagents and water. These incidents have resulted in fires, explosions, and chemical burns.
The highly reactive nature of iPrMgBr demands meticulous handling under anhydrous conditions. Proper storage in tightly sealed containers under an inert atmosphere is essential to prevent accidental exposure to moisture.
Standard operating procedures (SOPs) must explicitly address the dangers associated with this reaction. Comprehensive training for all personnel handling these chemicals is non-negotiable.
Mitigation and Prevention
Strict control of moisture: All glassware and equipment must be thoroughly dried before use. Anhydrous solvents should be used, and reactions should be performed under an inert atmosphere (e.g., nitrogen or argon).
Proper waste disposal: Grignard reagents must be quenched with a controlled protic source (e.g., saturated ammonium chloride solution) *before* disposal. Never dispose of unreacted Grignard reagents directly into aqueous waste streams.
Emergency procedures: Clear protocols for responding to spills or accidental exposure must be in place. Access to fire extinguishers, safety showers, and eyewash stations is crucial.
Regulatory Oversight and Future Actions
Regulatory agencies, such as OSHA (Occupational Safety and Health Administration), are increasingly focused on laboratory safety, including the handling of reactive chemicals. Labs must comply with relevant safety standards and guidelines.
Ongoing research is focused on developing safer alternatives to Grignard reagents. Furthermore, improved methods for handling and quenching reactive organometallic compounds are being explored.
Immediate action is required to review and update safety protocols in all laboratories using Isopropyl Magnesium Bromide. Prioritizing safety and preventing future incidents is paramount.