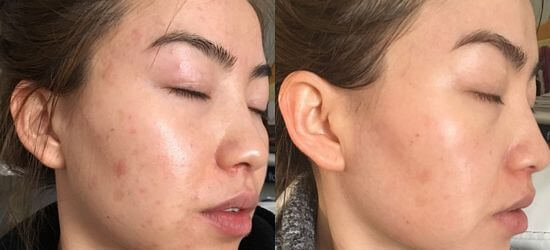
Mena Whitening Cream Before And After
URGENT WARNING: Health officials have issued a nationwide alert regarding Mena Whitening Cream following a surge in reported adverse reactions, including severe skin damage and potential long-term health consequences.
The cream, marketed for its skin-lightening properties, has been linked to serious side effects, prompting immediate action from regulatory bodies.
Adverse Reactions Surge
The Food and Drug Administration (FDA) has received a significant increase in reports of adverse reactions associated with Mena Whitening Cream over the past six months.
Reports include cases of severe skin irritation, burns, permanent discoloration, and even potential mercury poisoning.
WHO: Affected individuals range in age from 18 to 55, with a majority being female consumers.
What is Mena Whitening Cream?
Mena Whitening Cream is a topical product widely available online and in some retail outlets, marketed for its skin-lightening and brightening effects.
It often contains ingredients such as hydroquinone and corticosteroids, which can cause significant harm if used improperly or without medical supervision.
WHAT: The cream is being flagged for containing dangerous levels of prohibited substances.
Where and When?
The product is primarily sold online through various e-commerce platforms and social media channels, making it accessible nationwide and internationally.
WHERE: The product is primarily sold online. WHEN: The surge in reports began in early 2023.
Health Risks Detailed
Dermatologists warn that prolonged use of Mena Whitening Cream can lead to a range of health problems.
These include irreversible skin damage, increased risk of skin cancer, and systemic absorption of harmful chemicals.
The presence of mercury, often found in unregulated skin-lightening products, poses a serious threat to the kidneys and nervous system.
Symptoms to Watch For
Consumers are urged to immediately discontinue use of the cream and seek medical attention if they experience any of the following symptoms:
Severe burning or itching, blistering, swelling, changes in skin pigmentation, or signs of mercury poisoning such as tremors or cognitive issues.
FDA Action and Recall Information
The FDA has issued an official warning and is working to identify and remove the product from circulation.
Consumers who have purchased Mena Whitening Cream are advised to dispose of it immediately and report the purchase to the FDA.
HOW: The FDA is collaborating with online retailers to remove listings and prevent further sales.
Moving Forward
Authorities are intensifying efforts to track down the source of the contaminated cream and prosecute those responsible for its distribution.
The public is urged to exercise extreme caution when purchasing skin-lightening products and to consult with a dermatologist before using any new skincare treatments.
Ongoing investigations are focused on identifying other potentially harmful products on the market and strengthening regulations to protect consumers.


















