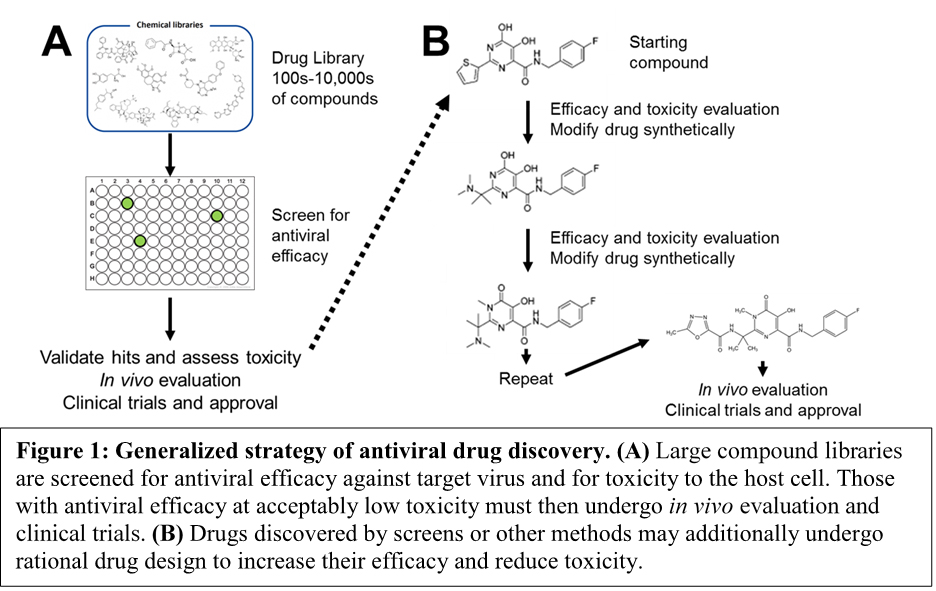
What Are Some Challenges Of Developing Antiviral Medications

The rapid spread of viral outbreaks, from influenza to COVID-19, underscores the urgent need for effective antiviral medications. However, developing these drugs presents a unique set of scientific and logistical hurdles.
Creating antivirals is a race against evolution. It's a complex endeavor fraught with challenges ranging from viral mutation to drug resistance and toxicity, slowing down progress and demanding innovative approaches to combat existing and emerging viral threats.
The Moving Target: Viral Mutation
Viruses are notorious for their high mutation rates. This constant evolution allows them to rapidly develop resistance to antiviral drugs.
Influenza, for example, mutates so quickly that new vaccines are required annually, and antiviral drugs like oseltamivir (Tamiflu) can lose effectiveness over time, according to a report by the World Health Organization (WHO).
This necessitates a continuous cycle of drug development and adaptation, a costly and time-consuming process.
Selectivity and Toxicity: Hitting the Right Target
Antiviral drugs must be highly selective, targeting viral processes without harming host cells. This selectivity is difficult to achieve.
Many early antivirals were plagued by significant toxicity, limiting their clinical use, as noted in a 2018 review published in the journal Antiviral Research.
Developing drugs that can selectively inhibit viral replication without causing unacceptable side effects remains a major challenge.
The Challenge of Broad-Spectrum Antivirals
While some antivirals are effective against a specific virus, like acyclovir for herpes simplex virus (HSV), the need for broad-spectrum antivirals that can target multiple viruses is critical.
Developing these broad-spectrum drugs is exceedingly difficult because viruses vary significantly in their structure and replication mechanisms.
Researchers at the National Institute of Allergy and Infectious Diseases (NIAID) are actively pursuing research into conserved viral targets that could be exploited for broad-spectrum antiviral development.
Drug Delivery: Getting the Medicine Where It's Needed
Effective drug delivery is crucial for antiviral efficacy. The drug must reach the site of viral replication in sufficient concentrations.
This can be particularly challenging for viruses that infect specific tissues or organs, such as the hepatitis C virus (HCV) which primarily infects the liver.
Nanoparticle-based drug delivery systems are being explored to improve targeted delivery of antiviral drugs, according to a 2022 study in Nature Nanotechnology.
The Need for Rapid Development and Deployment
Emerging viral outbreaks, such as the COVID-19 pandemic, highlight the urgent need for rapid antiviral development and deployment.
Traditional drug development timelines, which can span several years, are often too slow to effectively respond to these crises.
Accelerated drug development pathways, such as those utilized during the Ebola outbreak in 2014, are essential for addressing emerging viral threats.
Ethical Considerations in Antiviral Research
Ethical considerations are paramount in antiviral research, particularly during clinical trials. Ensuring patient safety and informed consent are crucial.
The rapid development and deployment of COVID-19 vaccines raised numerous ethical questions about trial design, access, and equitable distribution.
Robust ethical frameworks are necessary to guide antiviral research and ensure responsible innovation, according to guidelines from the U.S. Food and Drug Administration (FDA).
The Cost of Innovation
The development of antiviral drugs is a costly endeavor. The research, development, and clinical testing phases require significant investment.
This cost can be a barrier to entry for smaller companies and academic institutions, hindering innovation. Public-private partnerships, such as those facilitated by the Biomedical Advanced Research and Development Authority (BARDA), are crucial for supporting antiviral development.
Ensuring that antiviral drugs are affordable and accessible, particularly in low-income countries, is a critical challenge, according to a 2023 report by The Lancet.
Moving Forward: A Multi-pronged Approach
Overcoming the challenges of antiviral development requires a multi-pronged approach. This includes investing in basic research, developing new drug discovery technologies, and fostering collaborations between academia, industry, and government agencies.
The ongoing research into mRNA vaccines, as exemplified by the success of COVID-19 vaccines, demonstrates the potential of innovative approaches to combat viral diseases.
Continued investment and innovation are essential to ensure that we are prepared for future viral threats, according to recent statements by Dr. Anthony Fauci, former director of NIAID.