What Is True About Competitive Inhibitors

Imagine a bustling marketplace. Two merchants, both eager to sell their wares, stand side-by-side. One, the established vendor, is always there. The other, a newcomer, occasionally steps in front, briefly diverting potential customers. This everyday scenario is surprisingly similar to the microscopic world of enzymes and inhibitors, particularly competitive inhibitors, and their role within our very cells.
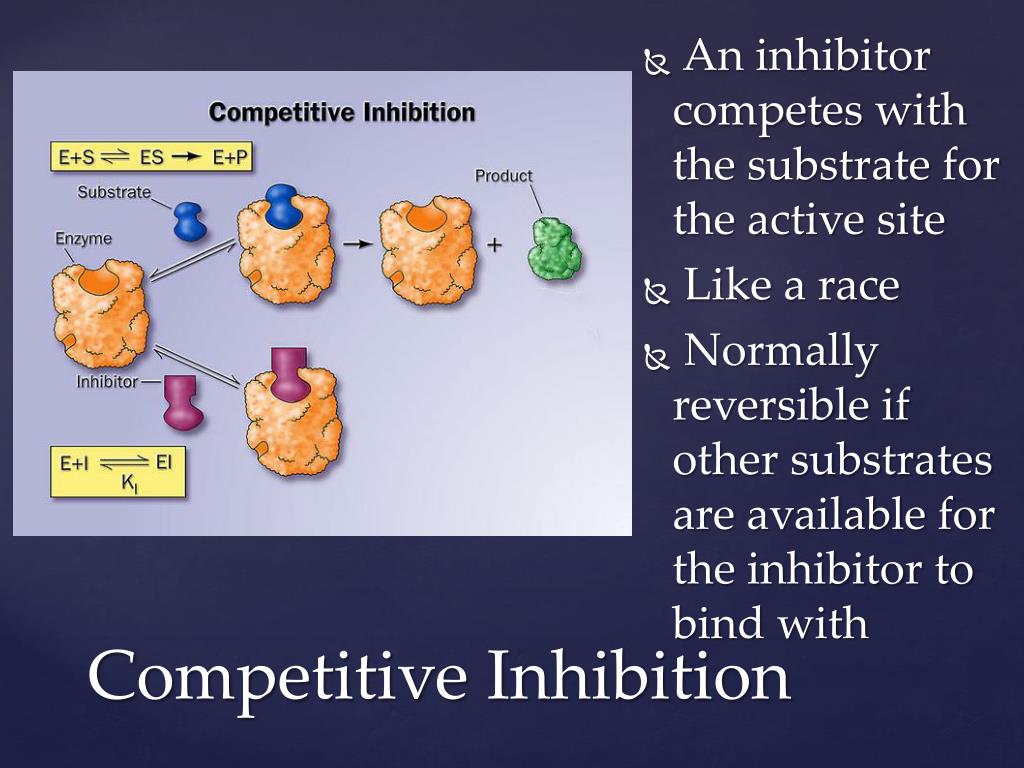
At the heart of countless biological processes, enzymes act as catalysts, speeding up reactions essential for life. Competitive inhibitors are molecules that interfere with these enzymes, specifically by competing for the same active site as the enzyme's normal target, the substrate.
The Lock and Key: Understanding Enzymes and Substrates
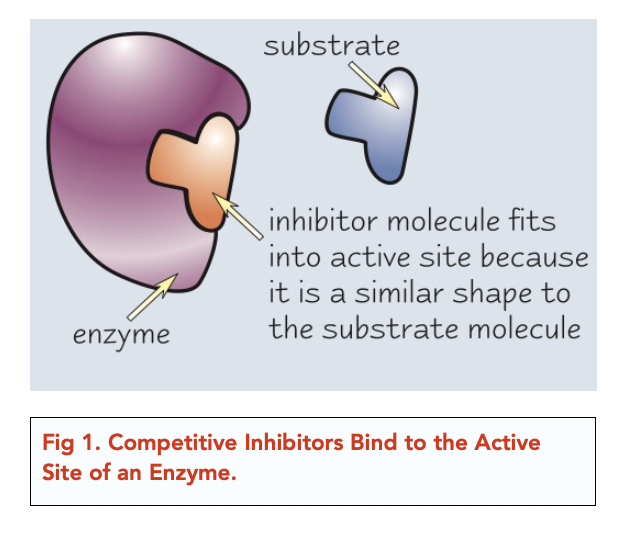
To truly understand competitive inhibition, we need to first grasp the basics of how enzymes function. Think of an enzyme as a specialized lock and the substrate as the key. The substrate fits precisely into the enzyme's active site, a region specifically shaped to bind with the substrate.
Once the substrate binds, the enzyme facilitates a chemical reaction, transforming the substrate into a product. Then, the product is released, and the enzyme is free to repeat the process. It's a beautifully efficient system.
A Biochemical Tug-of-War
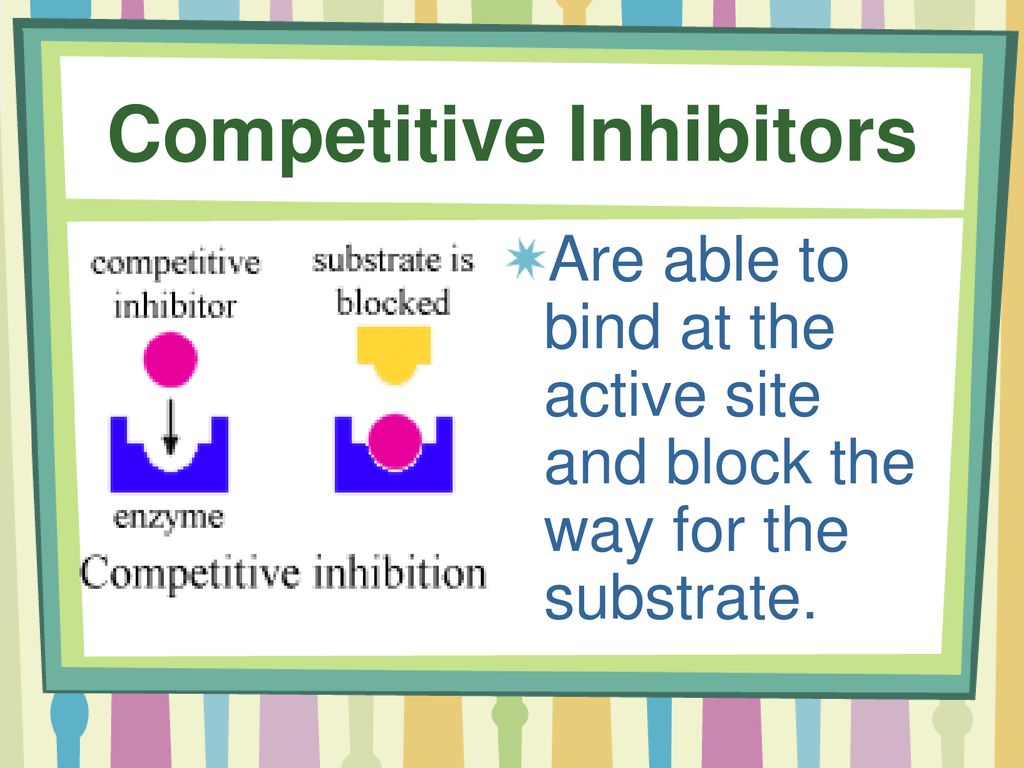
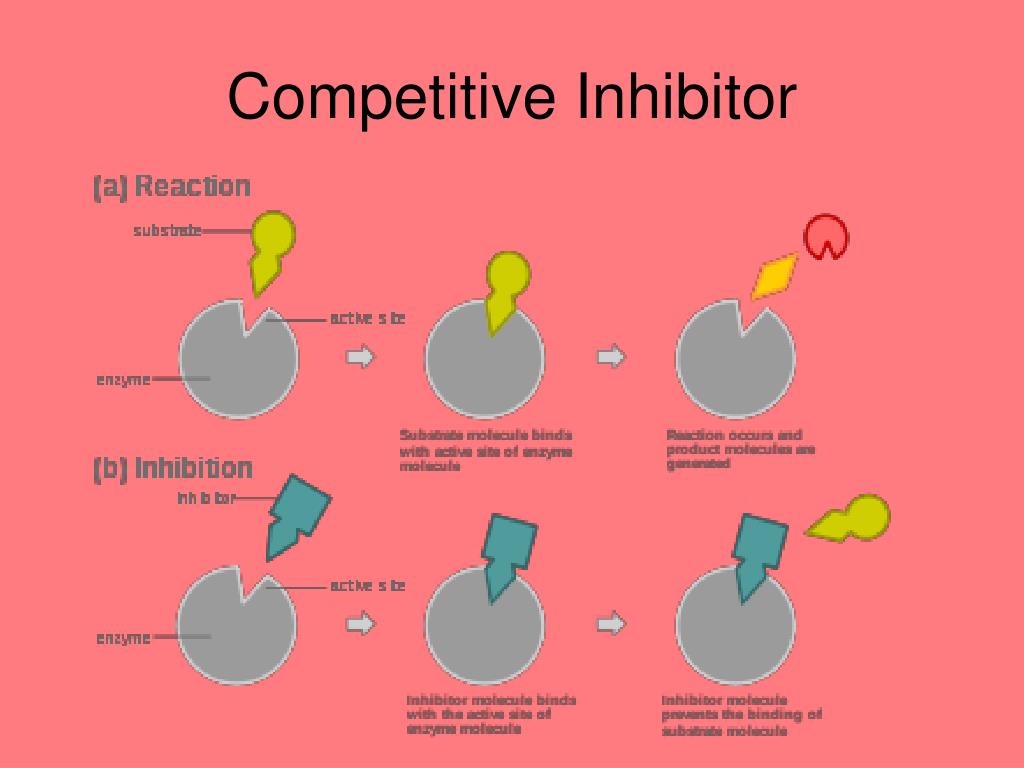
However, what happens when a "fake key," a competitive inhibitor, tries to fit into the lock? This is precisely what competitive inhibition is all about. The inhibitor, bearing a structural resemblance to the substrate, competes for the active site.
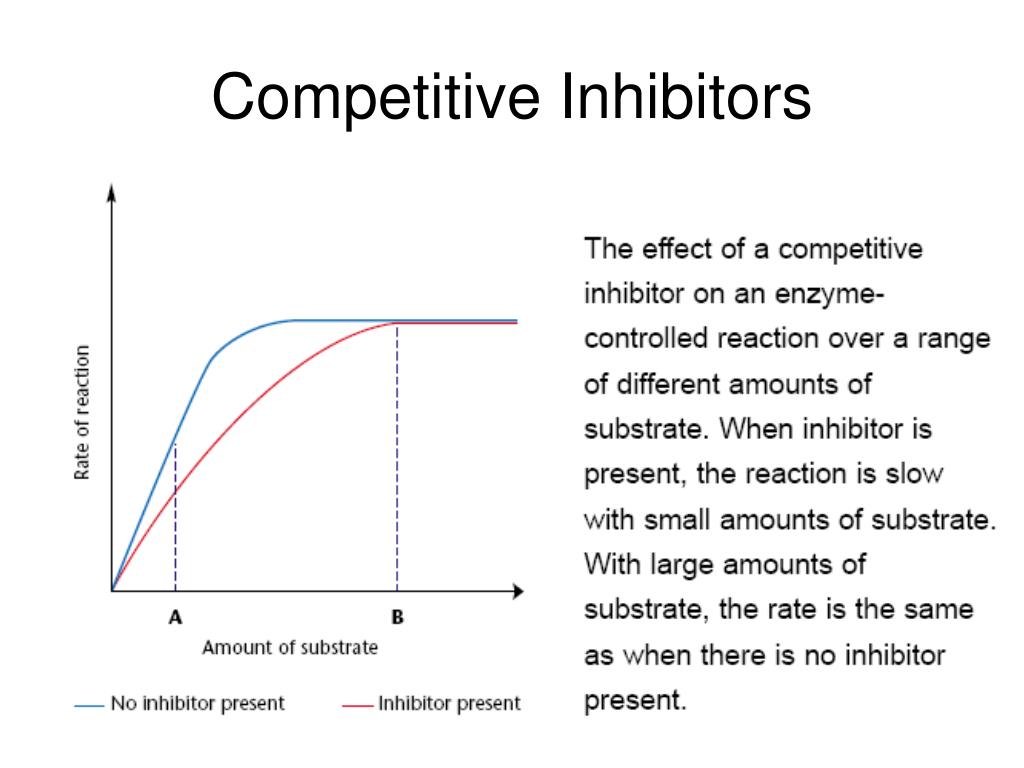
If the inhibitor successfully binds, it blocks the substrate from entering, effectively slowing down the reaction. It is important to remember that the inhibitor does not permanently disable the enzyme.
The binding is reversible, and the inhibitor can eventually detach, freeing the enzyme to bind with the substrate again.The key here is concentration.
Concentration is Key
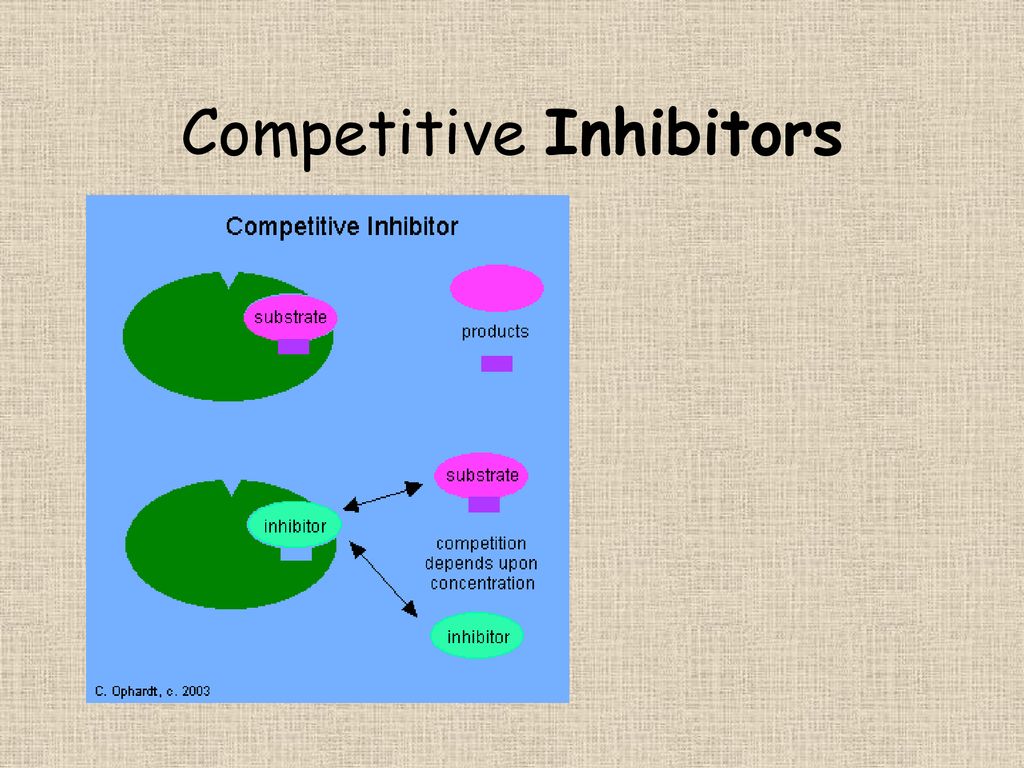
The degree to which a competitive inhibitor affects enzyme activity depends on the relative concentrations of the inhibitor and the substrate. If the substrate concentration is significantly higher than the inhibitor concentration, the substrate is more likely to bind to the enzyme.
In this scenario, the inhibitor's effect is minimal. Conversely, if the inhibitor concentration is high, it will effectively outcompete the substrate, leading to a more significant reduction in enzyme activity. This highlights the dynamic equilibrium at play.
Overcoming Inhibition: Flooding the System
A defining characteristic of competitive inhibition is that it can be overcome by increasing the substrate concentration. By adding more and more "keys" (substrate), we increase the likelihood that the substrate will find and bind to the enzyme before the inhibitor does.
This is a crucial point that distinguishes competitive inhibition from other types of enzyme inhibition, such as non-competitive inhibition, where increasing the substrate concentration has no effect.
Real-World Examples: Competitive Inhibition in Action
Competitive inhibition isn't just a theoretical concept; it plays a vital role in various biological and pharmaceutical contexts. Many drugs function as competitive inhibitors, targeting specific enzymes involved in disease processes. Methotrexate, for example, is a drug used in chemotherapy.
Methotrexate acts as a competitive inhibitor of dihydrofolate reductase, an enzyme crucial for DNA synthesis in rapidly dividing cells, such as cancer cells. By blocking this enzyme, methotrexate effectively slows down cancer cell growth.
Another example is the treatment of ethylene glycol poisoning
Ethylene glycol, found in antifreeze, is metabolized by alcohol dehydrogenase to toxic compounds. Ethanol is administered as a competitive inhibitor to block the metabolism of ethylene glycol.
By doing so, the ethylene glycol is excreted unchanged, preventing the formation of toxic metabolites and minimizing damage.
Distinguishing Competitive Inhibition from Other Types
It's important to differentiate competitive inhibition from other forms of enzyme inhibition, such as non-competitive and uncompetitive inhibition. In non-competitive inhibition, the inhibitor binds to a site on the enzyme that is different from the active site.
This binding alters the enzyme's shape, making it less effective at binding to the substrate, even if the substrate can still access the active site. Importantly, increasing the substrate concentration does not overcome non-competitive inhibition.
Uncompetitive inhibition, on the other hand, occurs when the inhibitor binds only to the enzyme-substrate complex, preventing the release of the product. The mode of binding sets each type apart, with consequential impact on its effects and possible workarounds.
Understanding the Kinetics: Michaelis-Menten and Lineweaver-Burk
Enzyme kinetics, the study of enzyme reaction rates, provides a quantitative framework for understanding competitive inhibition. The Michaelis-Menten equation describes the relationship between the reaction rate and the substrate concentration in the absence of inhibitors.
When a competitive inhibitor is present, the apparent Michaelis constant (Km) increases, indicating a lower affinity of the enzyme for the substrate. The maximum reaction rate (Vmax), however, remains unchanged, as the enzyme can still achieve its maximum rate if enough substrate is present to outcompete the inhibitor.
The Lineweaver-Burk plot, a double reciprocal plot of the Michaelis-Menten equation, provides a visual representation of these changes. In the presence of a competitive inhibitor, the Lineweaver-Burk plot shows an increase in the x-intercept (corresponding to an increase in Km) while the y-intercept (corresponding to 1/Vmax) remains the same.
The Significance of Studying Competitive Inhibition
Understanding competitive inhibition is crucial for various scientific disciplines, including biochemistry, pharmacology, and drug development. By understanding how inhibitors interact with enzymes, researchers can design more effective drugs that target specific disease-related enzymes.
Furthermore, studying competitive inhibition helps us understand the regulation of metabolic pathways and the complex interplay of enzymes and inhibitors within cells. It is a fundamental aspect of understanding cellular function.
Beyond Pharmaceuticals: Applications in Agriculture and Industry
The principles of competitive inhibition also extend beyond the realm of pharmaceuticals. In agriculture, inhibitors are used to control pests and weeds by targeting essential enzymes in these organisms.
In industrial processes, enzyme inhibitors can be used to regulate enzyme activity in various applications, such as food processing and biofuel production. The broad applicability underscores its importance.
Looking Ahead: Future Research and Applications
Research on competitive inhibition continues to evolve, with new discoveries constantly expanding our understanding of enzyme regulation. Scientists are exploring novel inhibitors with improved specificity and potency, aiming to develop more effective therapies for a wide range of diseases.
Advances in computational modeling and structural biology are also playing a key role, allowing researchers to predict and design inhibitors with greater precision. The future is bright with potential discoveries.
As we return to our bustling marketplace analogy, consider the constant dance between enzymes and competitive inhibitors within our bodies. It's a dynamic and intricate process, one that is essential for maintaining health and fighting disease. By unraveling the mysteries of competitive inhibition, we are gaining valuable insights into the fundamental processes of life and paving the way for innovative medical and technological advancements.


+inhibitor.jpg)