Which Areas Of An Antibody Determine Specificity

The ability of antibodies to precisely target and neutralize pathogens or aberrant cells is fundamental to the adaptive immune system and crucial for the success of countless diagnostic and therapeutic applications. But this exquisitely specific interaction isn't magic; it's a consequence of highly specialized regions within the antibody's structure, a topic of intensive research and ongoing discovery.
Delving into the molecular mechanisms that govern antibody specificity is paramount for designing more effective vaccines, developing targeted therapies for cancer and autoimmune diseases, and enhancing diagnostic tools. The "nut graf" of this story lies in the understanding that the hypervariable regions, also known as complementarity-determining regions (CDRs), located within the variable domains of both heavy and light chains, are the primary determinants of antigen specificity. Understanding these regions allows for tailored antibody engineering, paving the way for more potent and precise immunotherapeutic interventions.
The Antibody Structure: A Foundation for Specificity
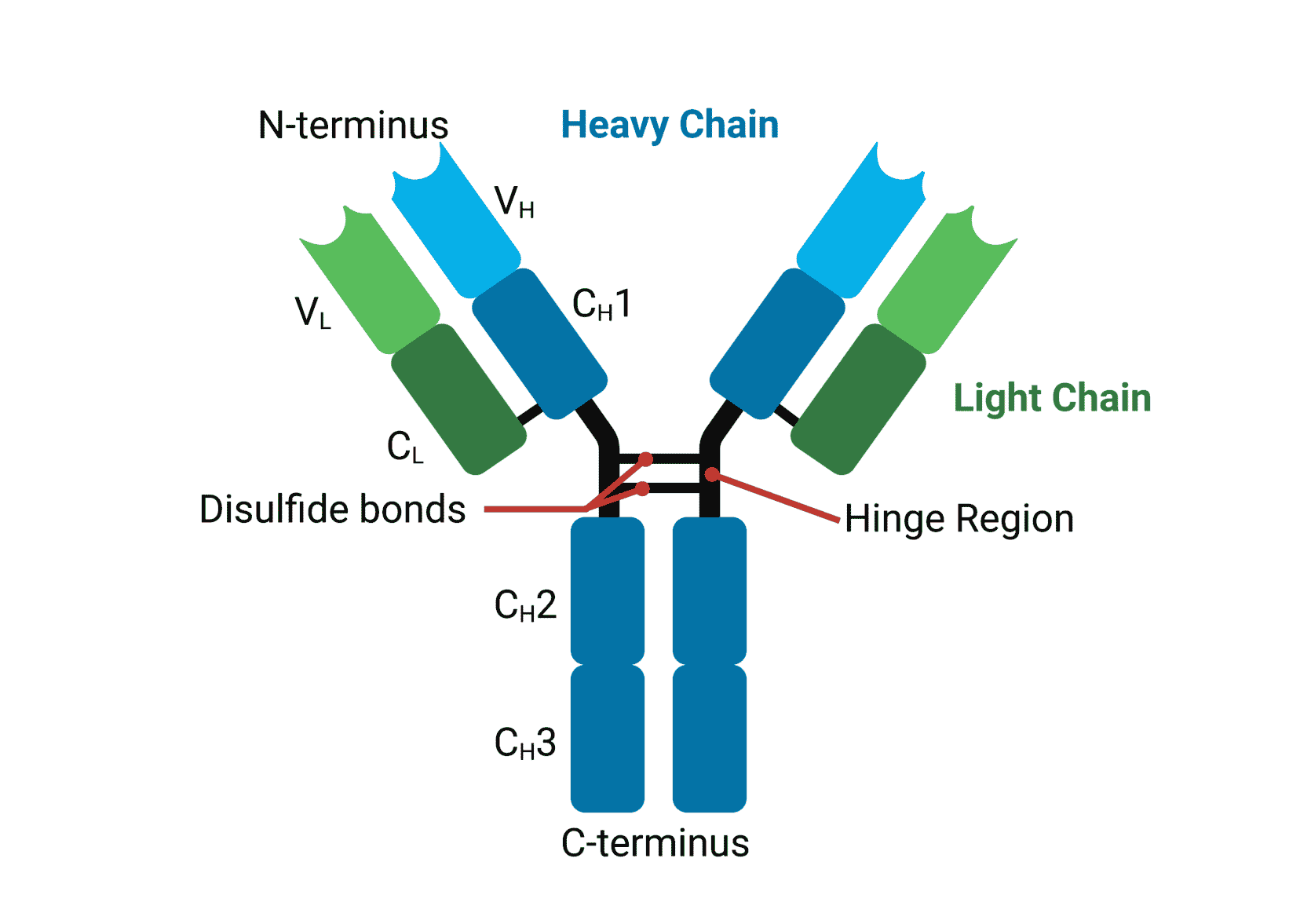
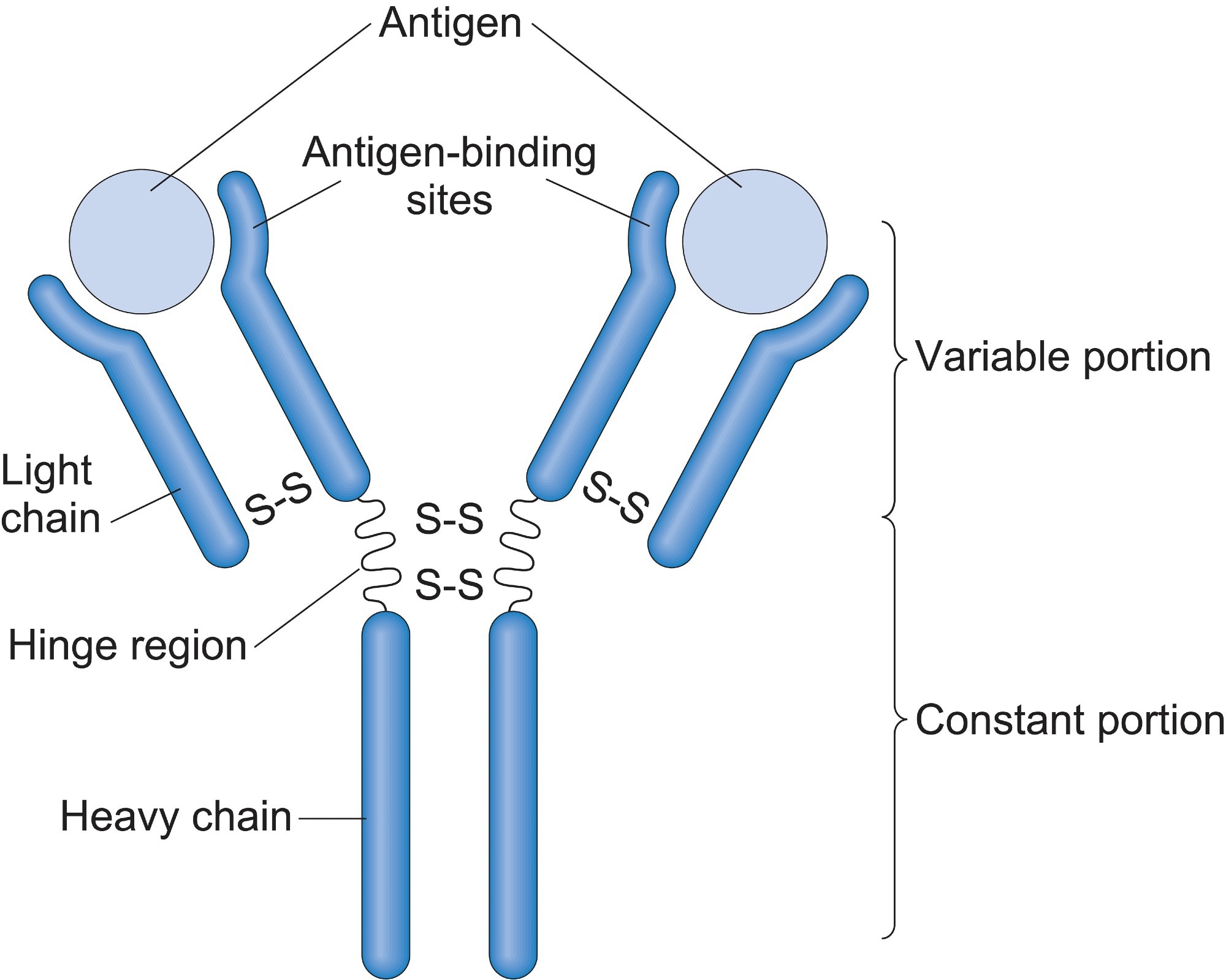
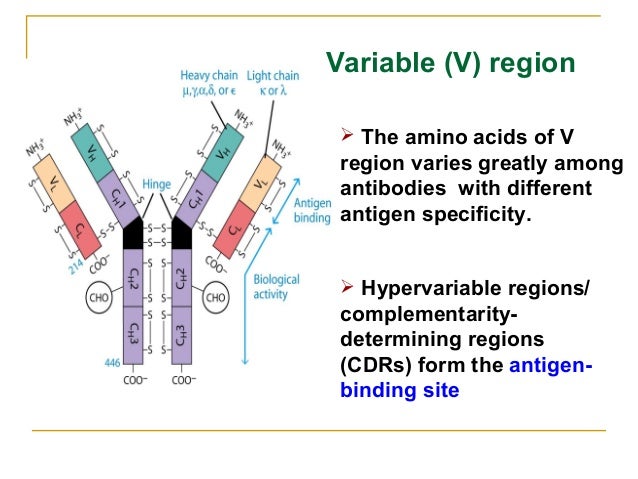
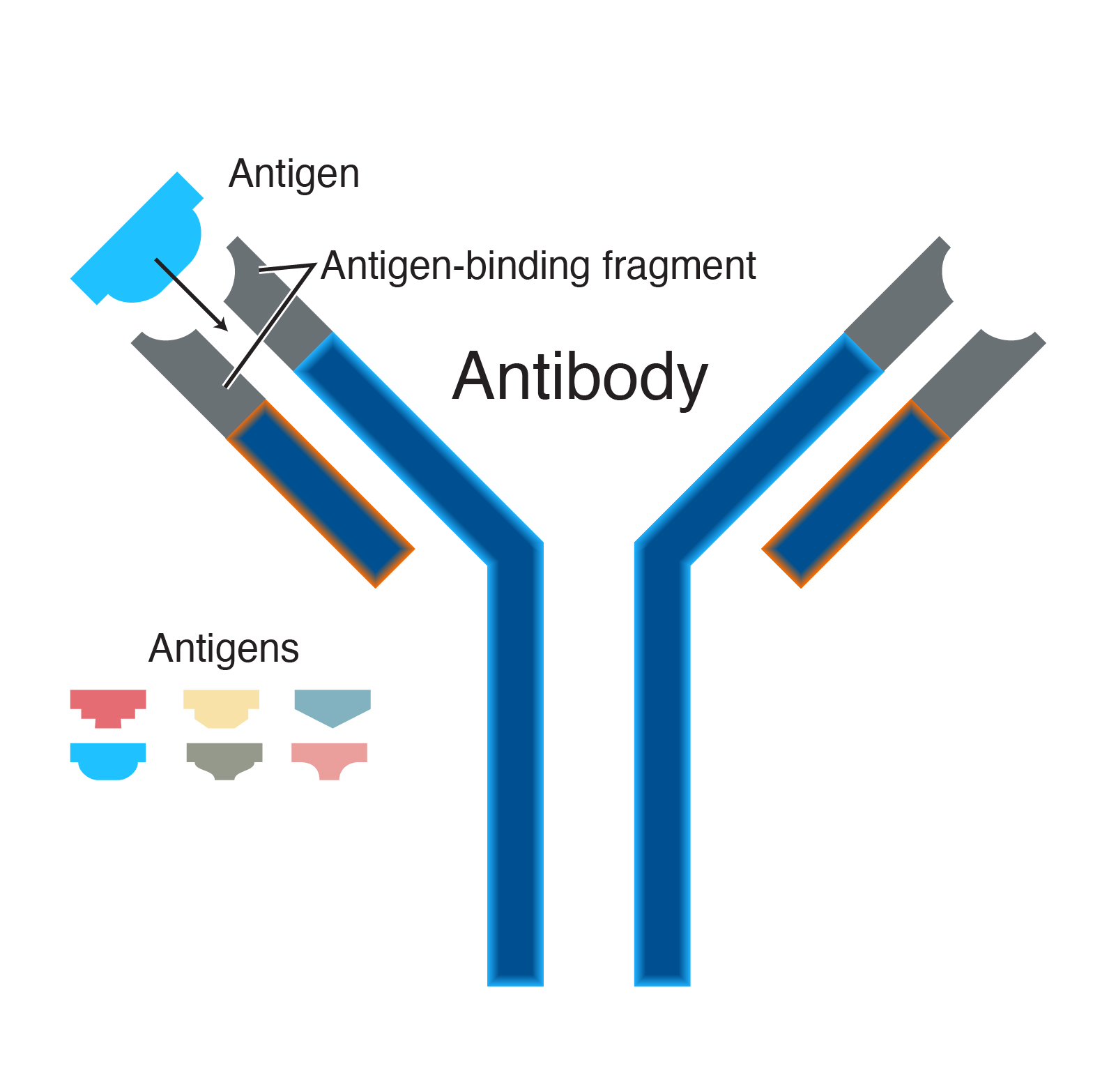
Antibodies, also known as immunoglobulins, are Y-shaped glycoproteins composed of two heavy chains and two light chains. Each chain has a variable region (V) and a constant region (C). The variable regions, specifically the VH (variable heavy) and VL (variable light) domains, are responsible for antigen binding.
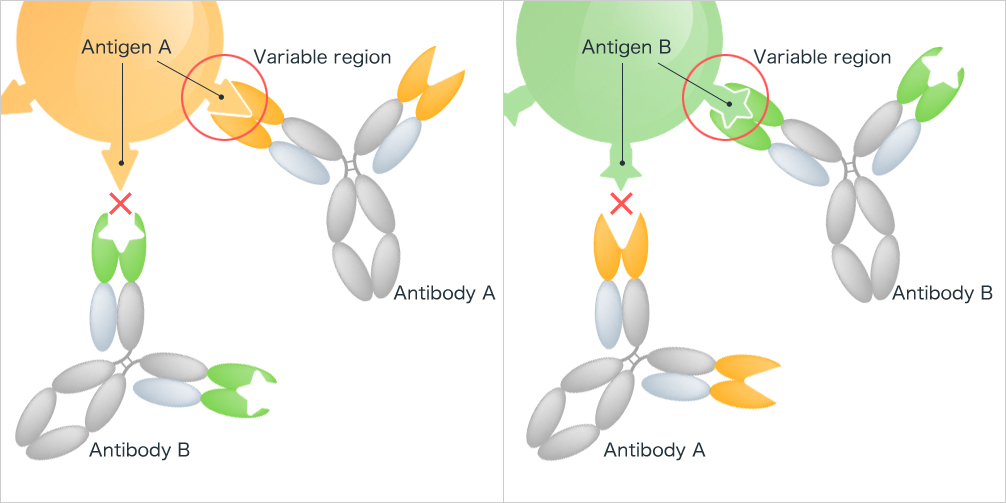
Within the VH and VL domains lie the all-important complementarity-determining regions (CDRs). These are short stretches of amino acids with immense variability. It is this variability that allows antibodies to recognize and bind to a vast array of antigens.
Complementarity-Determining Regions (CDRs): The Heart of Specificity
There are six CDRs in total: three located on the heavy chain (CDR-H1, CDR-H2, CDR-H3) and three on the light chain (CDR-L1, CDR-L2, CDR-L3). CDR3, particularly CDR-H3, often plays a dominant role in antigen recognition. This is due to its greater length and diversity compared to other CDRs.
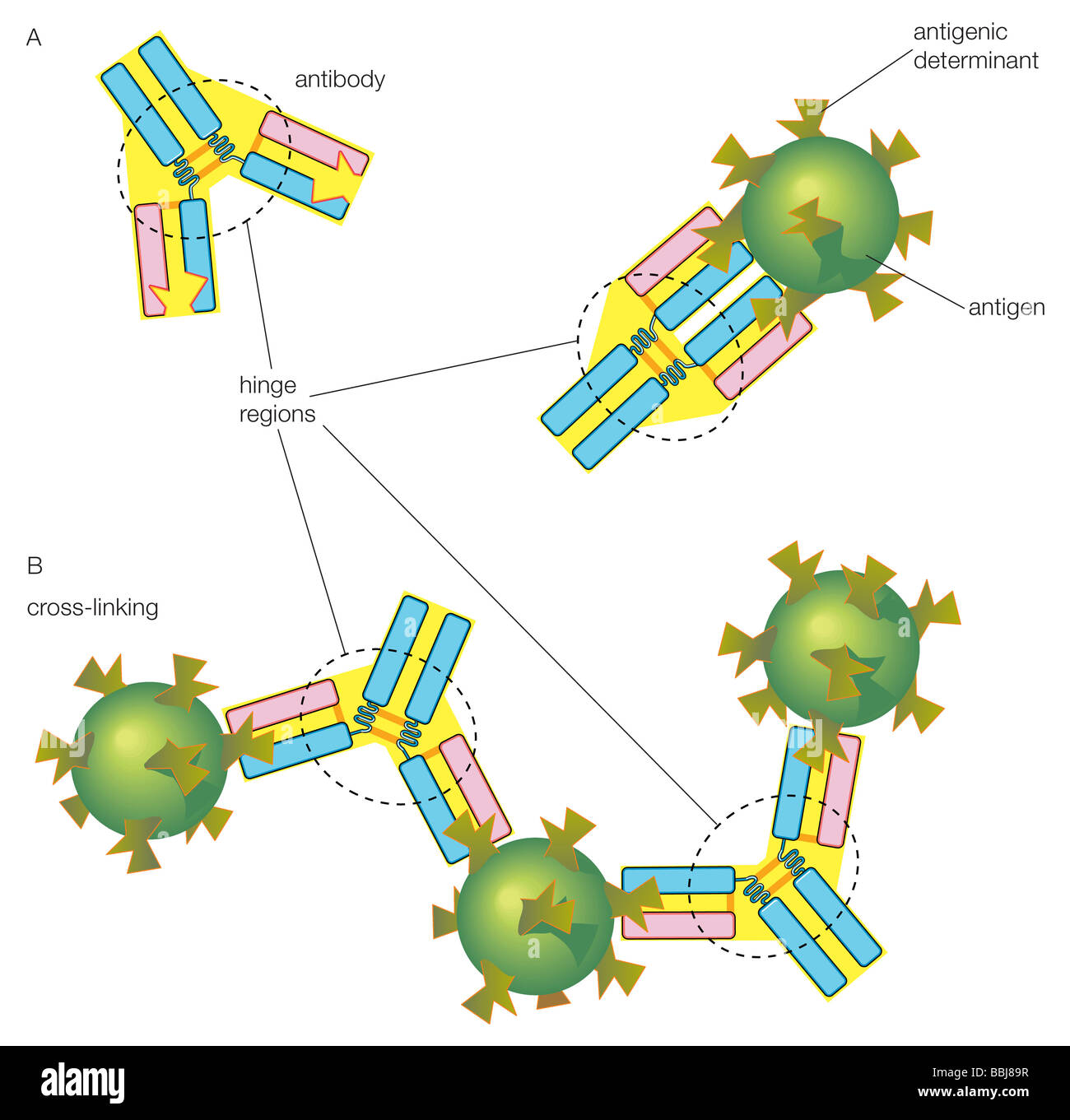
The amino acid sequence and three-dimensional structure of the CDRs dictate the shape and charge distribution of the antigen-binding site, also called the paratope. This paratope must be complementary to the shape and charge of a specific region on the antigen, known as the epitope. Think of it as a lock (antibody) and key (antigen) mechanism.
Affinity describes the strength of the interaction between a single antibody and its target antigen. Specificity, on the other hand, refers to the ability of an antibody to discriminate between different antigens and only bind to its intended target. CDRs are critical for both affinity and specificity.
The Role of Framework Regions
While the CDRs are the primary determinants of antigen specificity, the framework regions (FRs), which surround the CDRs, also contribute to the overall structure and stability of the antibody. The framework regions provide a scaffold that supports the CDRs and presents them in the correct orientation for antigen binding.
Changes in the framework regions can indirectly affect antigen binding by altering the conformation of the CDRs. Therefore, when engineering antibodies, it is crucial to consider the potential impact of mutations in both the CDRs and the framework regions.
Investigating Antibody Specificity: Experimental Approaches
Researchers employ a range of techniques to map the specific interactions between antibodies and their target antigens. X-ray crystallography can provide high-resolution structures of antibody-antigen complexes, revealing the precise amino acid contacts involved in binding.
Site-directed mutagenesis is used to systematically alter amino acids within the CDRs and assess the impact on antigen binding affinity and specificity. Surface plasmon resonance (SPR) and enzyme-linked immunosorbent assays (ELISAs) are also commonly used to quantify antibody-antigen interactions.
Computational modeling and bioinformatics are increasingly important for predicting antibody specificity and guiding antibody design. These approaches use algorithms to analyze antibody sequences and structures and predict their binding affinity to different antigens.
Antibody Engineering: Tailoring Specificity for Therapeutic Applications
The detailed understanding of antibody specificity has enabled the development of sophisticated antibody engineering techniques. CDR grafting involves transplanting CDRs from one antibody (typically a mouse antibody) onto the framework of a human antibody to reduce immunogenicity when used therapeutically in humans.
Affinity maturation is a process used to improve the binding affinity of an antibody for its target antigen. This can be achieved by introducing random mutations into the CDRs and selecting for antibodies with enhanced binding. These techniques are crucial for optimizing antibody-based therapies.
Bispecific antibodies, which can bind to two different antigens simultaneously, are another exciting area of antibody engineering. Bispecific antibodies are typically engineered to have two distinct sets of CDRs, each specific for a different target.
The Future of Antibody Specificity Research
Research into antibody specificity is ongoing. Scientists are constantly seeking to better understand the complex interplay of factors that govern antigen recognition.
Future directions include developing more sophisticated computational models for predicting antibody specificity, exploring the role of glycosylation (sugar modifications) on antibody function, and developing novel antibody formats with enhanced therapeutic properties.
Ultimately, a deeper understanding of antibody specificity will lead to the development of more effective and targeted therapies for a wide range of diseases. This has the potential to significantly improve human health.