Choose The Compound That Is The Most Soluble In Water

The seemingly simple question of which compound boasts the highest solubility in water has ignited a complex and crucial debate across scientific disciplines. From pharmaceutical development to environmental remediation, understanding solubility dictates how substances interact within aqueous environments, influencing everything from drug delivery to pollutant dispersal. This seemingly academic exercise holds profound real-world implications, demanding precise measurement and a nuanced understanding of intermolecular forces.
At the heart of this debate lies the intricate interplay between molecular structure, polarity, and temperature. Determining the most water-soluble compound isn't just about identifying a single winner. It is about understanding the fundamental principles that govern these interactions, ultimately leading to more effective solutions across a myriad of scientific and industrial challenges. The implications stretch from optimizing drug formulations to mitigating environmental contamination, making this a field of continuous and critical investigation.
The Contenders: A Look at Key Water-Soluble Compounds
While identifying a single "most soluble" compound proves elusive due to varying experimental conditions and measurement techniques, certain compounds consistently rank high in water solubility tests.
Ionic Compounds: A Strong Start
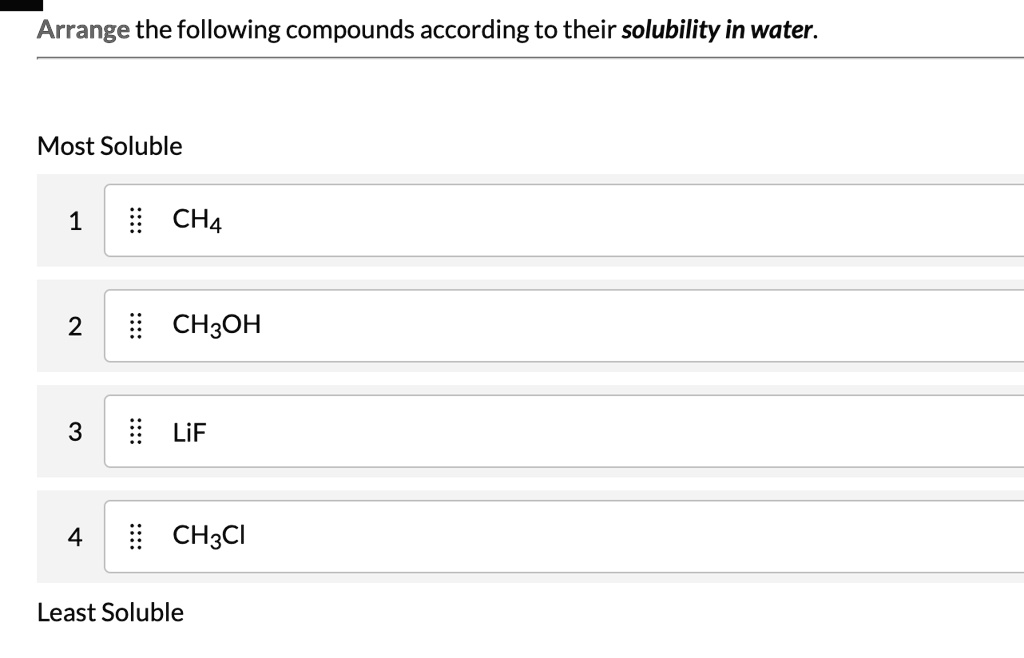
Ionic compounds, especially those containing alkali metals or nitrate ions, generally exhibit high water solubility. This solubility stems from the strong electrostatic interactions between water molecules and the charged ions, breaking the ionic lattice structure.
For example, Sodium Chloride (NaCl), common table salt, dissolves readily in water, demonstrating the power of ion-dipole interactions. However, not all ionic compounds are highly soluble; some, like Silver Chloride (AgCl), have very low solubility due to stronger lattice energies.
Polar Organic Compounds: Harnessing Hydrogen Bonding
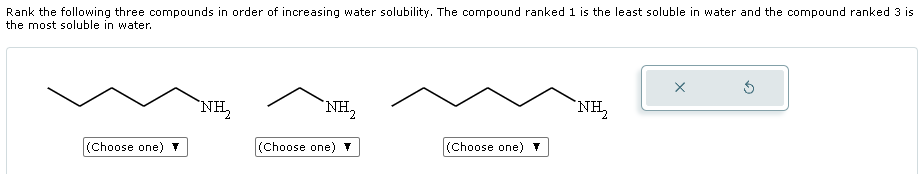
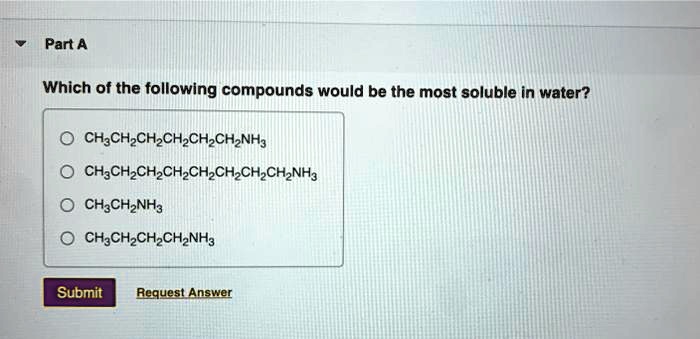
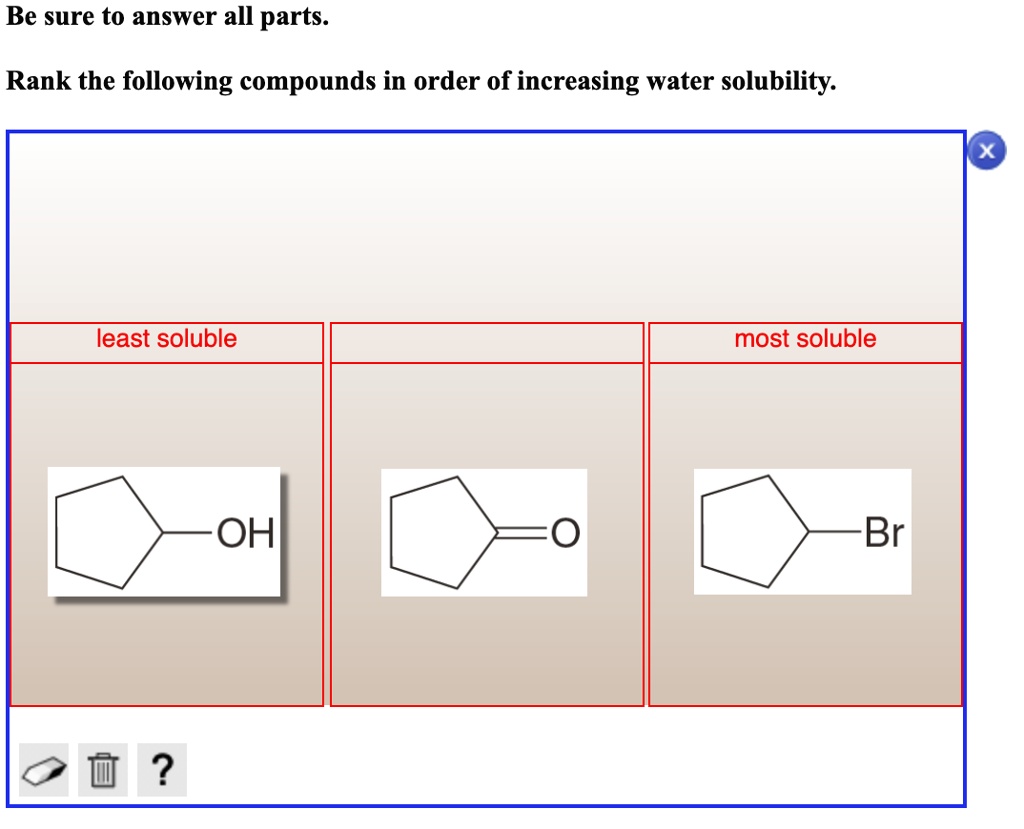
Many polar organic compounds containing hydroxyl (-OH) or amine (-NH2) groups exhibit good water solubility due to their ability to form hydrogen bonds with water molecules. These bonds enhance the interaction between the compound and water, facilitating dissolution.
Ethanol (C2H5OH), a simple alcohol, is completely miscible with water, meaning it can dissolve in water in any proportion. This miscibility highlights the effectiveness of hydrogen bonding in promoting solubility.
Sugars: A Complex Case of Hydrogen Bonding
Sugars, like glucose (C6H12O6) and sucrose (table sugar), are well-known for their high water solubility. The many hydroxyl groups present in sugar molecules allow for extensive hydrogen bonding with water.
This extensive hydrogen bonding overcomes the intermolecular forces holding the sugar molecules together, allowing them to disperse throughout the water. However, the solubility of different sugars can vary significantly depending on their specific structure and configuration.
Challenges in Determining the "Most" Soluble
Defining the “most soluble” compound is not straightforward due to several factors. These include variations in temperature, pressure, and the presence of other solutes.
Solubility is temperature-dependent; generally, the solubility of solids increases with temperature, but exceptions exist. Pressure also plays a role, particularly for gases dissolved in water.
Furthermore, the presence of other compounds in the water can affect the solubility of the target compound, a phenomenon known as the common ion effect. It’s a reminder that water doesn't exist in isolation.
The Role of Research and Experimentation
Precise determination of water solubility requires rigorous experimental techniques. Scientists employ various methods, including gravimetric analysis, spectrophotometry, and electrochemical measurements.
These methods allow for accurate quantification of the amount of compound dissolved in a given volume of water at a specific temperature and pressure. Standardized procedures and calibrated instruments are crucial for obtaining reliable and reproducible results.
Organizations like the National Institute of Standards and Technology (NIST) play a vital role in developing and maintaining reference materials and standards for solubility measurements. These standards ensure consistency and comparability across different laboratories and studies.
Implications and Applications of Solubility Knowledge
Understanding water solubility is paramount across diverse fields.
In pharmaceuticals, solubility influences drug absorption, bioavailability, and efficacy. Formulating drugs with optimal solubility is crucial for ensuring that they reach their target sites in the body and exert their therapeutic effects.
In environmental science, solubility governs the transport and fate of pollutants in aquatic ecosystems. Knowing the solubility of contaminants is essential for predicting their movement, accumulation, and potential impact on water quality and aquatic life.
Looking Ahead: Future Directions in Solubility Research
The quest to understand and predict water solubility continues. Researchers are developing advanced computational models and experimental techniques.
These models aim to predict solubility based on molecular structure and intermolecular interactions. They reduce the need for extensive experimental testing.
Furthermore, the development of novel solvents and solubilization techniques promises to enhance the solubility of poorly soluble compounds. It will open new avenues for drug delivery and environmental remediation. Ultimately, continuous research efforts are necessary to refine our understanding of this fundamental property and unlock its full potential.
"Solubility is not just a physical property; it's a key that unlocks the door to understanding how molecules interact with each other and with the environment around them," - Dr. Anya Sharma, Leading Solubility Researcher.