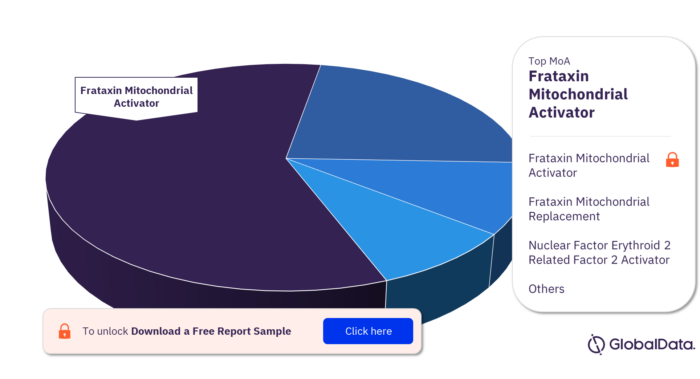
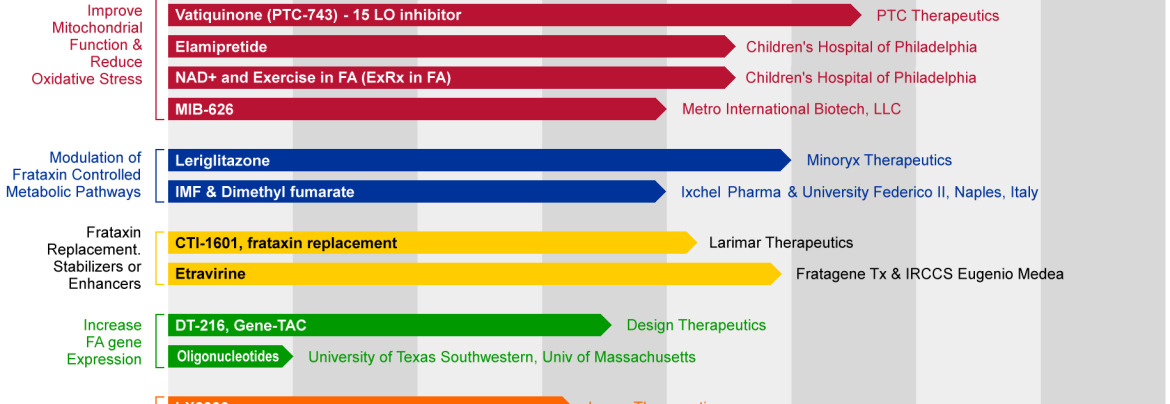
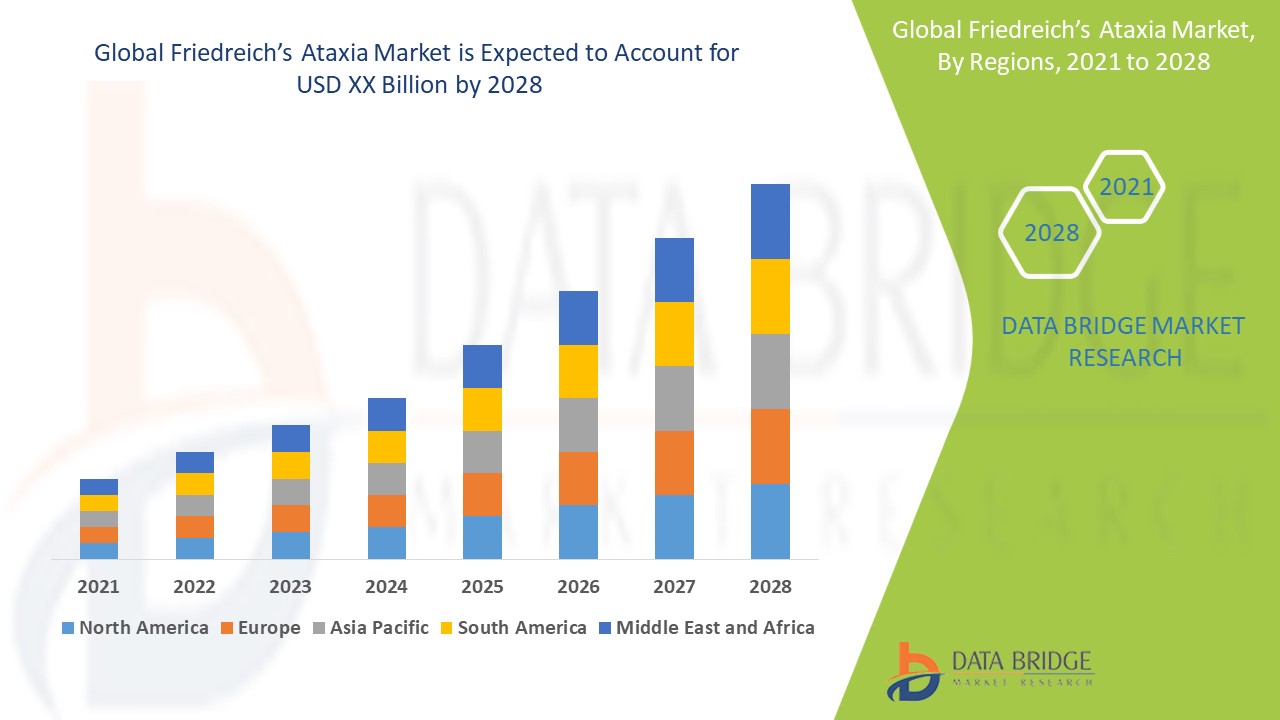
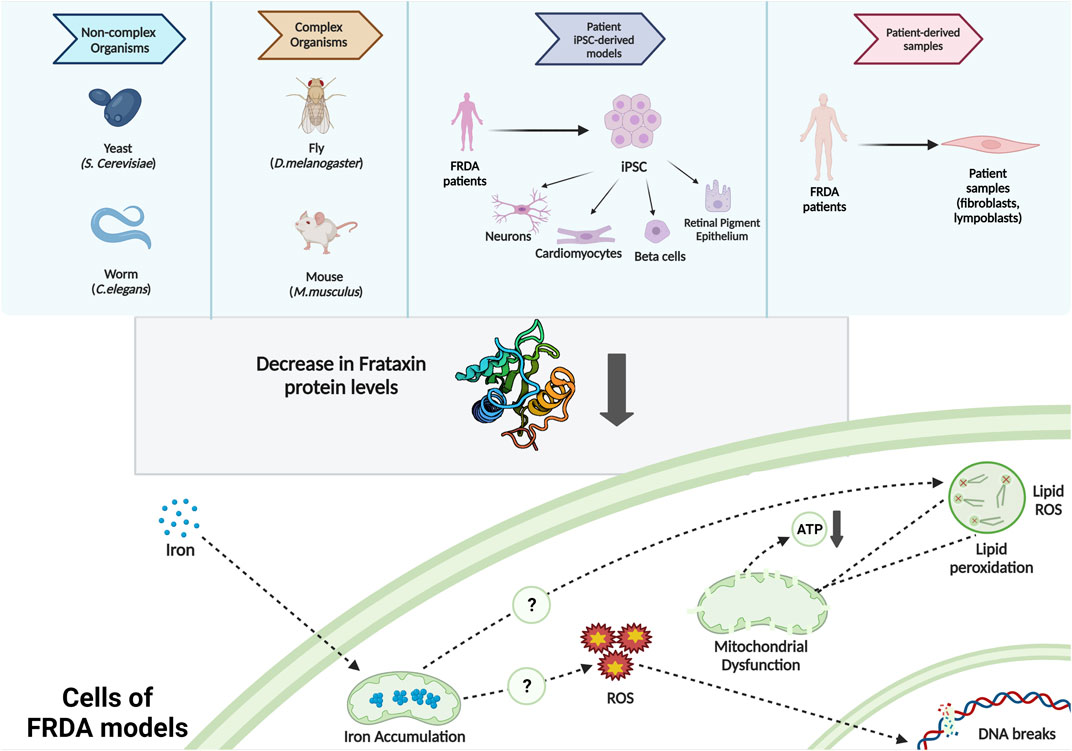
Friedreich Ataxiamarketed And Pipeline Drugs Analysis
The landscape of Friedreich Ataxia (FA) treatment is undergoing a dramatic shift, with groundbreaking therapies reaching the market and a robust pipeline promising further advancements. This analysis provides a concise overview of approved drugs and investigational agents, highlighting their mechanisms, clinical trial data, and potential impact on patients.
Approved Therapies: A New Dawn for FA Patients
For decades, treatment for FA focused solely on managing symptoms. However, the recent approval of disease-modifying therapies marks a pivotal moment.
Omaveloxolone (Skyclarys®)
Omaveloxolone, marketed as Skyclarys® by Reata Pharmaceuticals (now Biogen), is the first FDA-approved treatment specifically for Friedreich's Ataxia. It works by activating Nrf2, a transcription factor that improves mitochondrial function and reduces oxidative stress.
Approval was based on the pivotal MOXIe Part 2 trial, demonstrating a statistically significant improvement in neurological function as measured by the modified Friedreich Ataxia Rating Scale (mFARS).
Skyclarys® offers hope to patients by slowing disease progression, potentially improving quality of life. It is an oral medication taken daily.
Pipeline Drugs: Promising Candidates on the Horizon
Beyond Skyclarys®, a diverse range of therapeutic approaches are being investigated in clinical trials, targeting various aspects of FA pathology.
Gene Therapy Approaches
Multiple gene therapy programs are in development, aiming to deliver a functional copy of the FXN gene, which is deficient in FA patients. These therapies utilize adeno-associated viral (AAV) vectors.
Companies such as Lexeo Therapeutics and others are actively pursuing gene therapy, with early-stage clinical trials showing promising results. These approaches aim to correct the underlying genetic defect.
The hope is that a single administration of gene therapy could provide long-term benefit by restoring frataxin production.
FXN mRNA Therapy
Another avenue being explored is mRNA therapy, which involves delivering messenger RNA encoding for frataxin.
This approach, like gene therapy, aims to increase frataxin levels. PTC Therapeutics is among the companies developing FXN mRNA therapies.
mRNA therapies offer potential advantages in terms of safety and ease of manufacturing compared to gene therapy.
Mitochondrial-Targeted Therapies
Given the role of mitochondrial dysfunction in FA, several companies are developing therapies that target mitochondria directly.
These therapies aim to improve mitochondrial energy production and reduce oxidative stress. Clinical trials are underway to assess the efficacy of these compounds.
Improving mitochondrial function could have a significant impact on FA symptoms and disease progression.
Epigenetic Modulators
Epigenetic modulation is another area of active research, focusing on drugs that can alter gene expression.
These drugs aim to "unlock" the silenced FXN gene in FA patients, increasing frataxin production. Research is being conducted to identify the most effective epigenetic targets.
Epigenetic therapies offer a potentially reversible approach to treating FA.
Challenges and Future Directions
Despite the progress, significant challenges remain in FA drug development. These include the need for better biomarkers to track disease progression and treatment response.
Clinical trial design is also crucial, with efforts focused on developing more sensitive outcome measures. Another challenge is the heterogeneity of FA, which may require personalized treatment approaches.
Ongoing research is focused on addressing these challenges and developing more effective therapies for all FA patients.
Conclusion
The approval of Omaveloxolone (Skyclarys®) represents a major milestone for the FA community. Furthermore, the robust pipeline of investigational therapies offers continued hope for improved treatments.
Patients and families are encouraged to stay informed about clinical trials and consult with their healthcare providers to determine the most appropriate treatment options.
Continued investment in research and development is essential to further advance the field and ultimately find a cure for Friedreich's Ataxia.