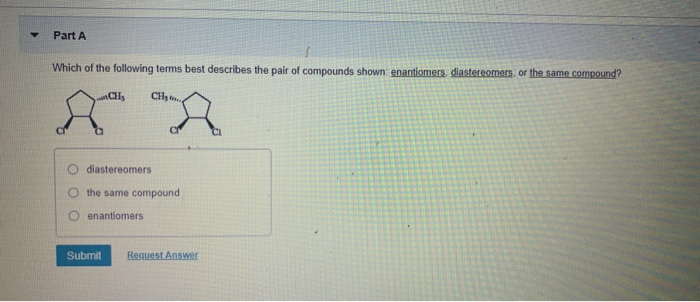
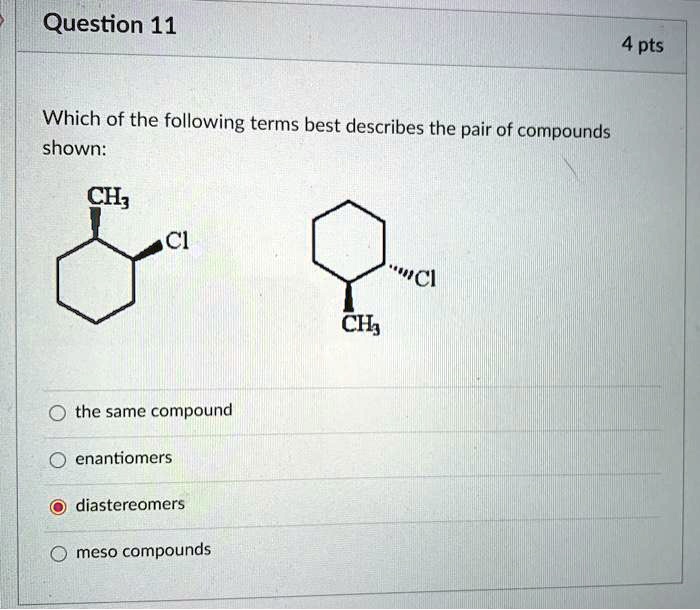
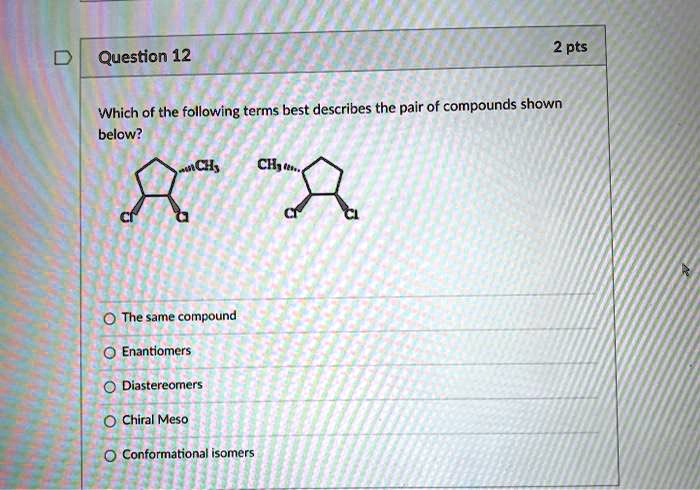
Which Of The Following Describes A Compound
Confusion reigns as test-takers nationwide grapple with a seemingly straightforward, yet deceptively complex question: "Which of the following describes a compound?" The seemingly simple query has triggered widespread debate and highlighted fundamental misunderstandings of basic chemistry.
This article breaks down the core elements of the question, providing clarity and definitive answers based on established scientific principles, addressing the wave of uncertainty and frustration impacting students and professionals alike.
Defining a Compound: The Essentials
At its most basic, a compound is a substance formed when two or more chemical elements are chemically bonded together. This bonding occurs through the sharing or transfer of electrons.
Crucially, a compound's properties are distinct from those of its constituent elements. Consider water (H2O): hydrogen and oxygen are both flammable gases, yet water is essential for extinguishing fires.
Key Characteristics of Compounds
Several key characteristics differentiate compounds from other substances. These include: Fixed Composition, Chemical Bonds, and Unique Properties.
Fixed Composition: A compound always contains the same elements in the same proportion by mass. For example, water is always two hydrogen atoms and one oxygen atom.
Chemical Bonds: Atoms in a compound are held together by chemical bonds, such as ionic or covalent bonds. These bonds require energy to break.
Unique Properties: As previously mentioned, a compound's properties are different from those of its constituent elements. This is a defining characteristic.
Common Misconceptions & Distractors
The confusion often stems from distinguishing compounds from mixtures. Mixtures are combinations of substances that are physically combined but not chemically bonded.
Unlike compounds, mixtures can be separated by physical means, such as filtration or evaporation. Air, for example, is a mixture of nitrogen, oxygen, and other gases.
Another source of error is confusing compounds with elements. An element is a pure substance consisting of only one type of atom, like gold (Au) or silver (Ag).
"The key difference lies in the bonding. Compounds are chemically bonded, while mixtures are merely physically combined," emphasizes Dr. Eleanor Vance, Professor of Chemistry at the Institute of Advanced Scientific Research.
Examples to Solidify Understanding
To further illustrate, let's examine some common examples: Sodium chloride (NaCl), also known as table salt, is a compound formed from sodium and chlorine.
Carbon dioxide (CO2) is a compound produced by bonding carbon and oxygen atoms. Methane (CH4), the primary component of natural gas, is another familiar compound.
These examples highlight the ubiquitous nature of compounds in our daily lives and the importance of understanding their fundamental properties.
How to Approach the Question
When faced with the question "Which of the following describes a compound?", look for options emphasizing chemical bonding and fixed composition. Eliminate options that suggest physical mixing or single-element substances.
Pay close attention to wording. Options containing phrases like "physically combined" or "can be separated by physical means" are likely distractors.
Remember the defining characteristic: the compound's properties are distinct from its constituent elements.
Expert Insights & Recommendations
Dr. Vance advises students to revisit basic chemistry principles. "A strong foundation in atomic structure, bonding, and the difference between elements, compounds, and mixtures is crucial," she states.
Furthermore, practice identifying compounds from their chemical formulas. This helps reinforce the concept of fixed composition and chemical bonding.
Consult reliable textbooks and online resources to clarify any remaining doubts.
Addressing the Fallout and Next Steps
The widespread confusion surrounding this seemingly simple question has prompted calls for curriculum review. Several educational institutions are re-evaluating their approach to teaching fundamental chemistry concepts.
Online forums and educational platforms are flooded with discussions and explanations, aiming to provide further clarity and support to those struggling with the question.
Efforts are underway to create more accessible and engaging learning materials to prevent similar misunderstandings in the future. A national task force has been created to specifically address this. Their first meeting is scheduled for November 8th.
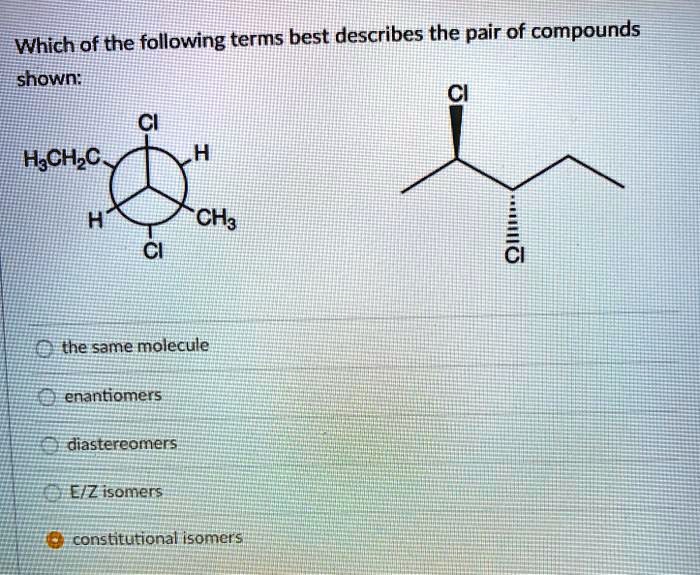




![Which Of The Following Describes A Compound [ANSWERED] 3 Which of the following statements describes two compounds](https://media.kunduz.com/media/sug-question-candidate/20220421170133759481-4338741.jpg?h=512)